Difference between revisions of "Major Processes in Organic Solar Cells"
Cmditradmin (talk | contribs) |
Cmditradmin (talk | contribs) |
||
| Line 89: | Line 89: | ||
===Charge Migration=== | ===Charge Migration=== | ||
[[Image:Opv19migration.JPG|thumb|300px|OPV Step | [[Image:Opv19migration.JPG|thumb|300px|OPV Step 4 Charge Migration]] | ||
Inorganic solar cells can be very efficient because a single inorganic material can be used with an exciton binding energy that is negligible at room temperature. The physics of organic solar cells is very different because the exciton is strongly bound. You need to have an acceptor and donor component with as much interface as possible between them in order that the formed excitons can quickly reach the interface to disassociate. | Inorganic solar cells can be very efficient because a single inorganic material can be used with an exciton binding energy that is negligible at room temperature. The physics of organic solar cells is very different because the exciton is strongly bound. You need to have an acceptor and donor component with as much interface as possible between them in order that the formed excitons can quickly reach the interface to disassociate. | ||
Revision as of 11:08, 8 July 2010
| Previous Topic | Return to OPV Menu | Next Topic |
Edmond Becquerel (1820-1891) was first to discover the photovoltaic effect. But it was 1954 before the first efficient cell was developed by Chapin, Fuller and Pearson in Bell Labs. They reported a silicon cell with 6% efficiency.
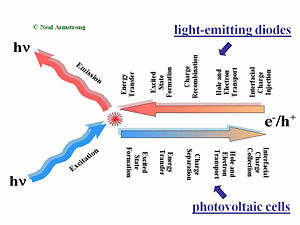
Comparison of OLEDs and OPVs
OLEDs produce light by the injection of charge carriers from the contacting electrodes. Holes typically are injected into the hole-transport layer (HTL) at the bottom transparent contact, and electrons are typically injected at the top metallic contact into the electron transport layer (ETL). These charge carriers migrate toward the center of the device under the influence of the applied field. The rates of charge migration are field-dependent, and dependent upon the mobilities of the holes in the HTL and the electrons in the ETL. In the center of the device, at the HTL/ETL interface, these injected charges (which reside on molecular species as radical cations and radical anions, or polaronic states), must find each other and recombine to form an excited (excitonic) state, from which emission occurs to create light. Exciton migration, energy transfer to dopants in the OLED, and energy losses occur afterward: the entire process from injection to emission of a photon occurs in less than one microsecond.
An organic photovoltaic cell works in a complementary fashion. Light is absorbed in either of two donor (D) or acceptor (A) layers, creating excitonic states which must diffuse to the D/A interface. Differences in ionization potential and electron affinities of D and A cause these excited states to dissociate into free charge carriers (electrons and holes residing on molecular species). The combination of diffusion and migration of these charge carriers to the collection electrodes, and the harvesting of these charges by these electrodes, produces a current in the external circuit as a specific voltage, the product of which is the power produced by the OPV.
Energy conversion in OPVs
Light Absorption
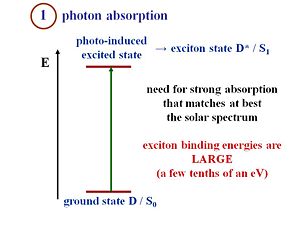
The most efficient OPVs currently have bandgap energies at or above 1.5 eV, which means that they are transparent to much of the near-IR and red regions of the visible wavelength spectrum. These are the regions of maximum solar flux, therefore there has been a great deal of attention focused on creating organic donor and acceptor layers with lower bandgaps, without sacrificing either chemical stability or photopotential (see below).
Inorganic semiconductors are better matched in their bandgap energies to the solar spectrum, but have lower absorptivities than organic materials, requiring thicker absorbing layers, and high purities (and high costs) to insure efficient operation.
Another key difference between OPVs and conventional inorganic solar cells is in the exciton binding energy. In both systems excitons (excited states) are formed upon photon absorption. In inorganic semiconductors the energy required to dissociate these excitons into charge carriers is quite small (a few milli-eVs, easily achieved at room temperature). In organic semiconductors the "exciton binding energy" can be as high as 0.5 eV or higher, requiring the formation of a D/A heterojunction (see below) to provide the internal electrochemical driving force for exciton dissociation to occur.
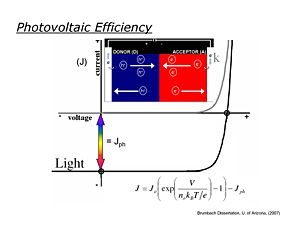
Photovoltaic Efficiency
Ideally photovoltaic devices behave like diodes, with dark current/voltage (J/V) curves following the Shockley equation. In the dark in the reverse bias direction little measurable current flows, whereas in the forward bias direction, current increases exponentially with applied voltage. When the diode is illuminated, the J/V curve is ideally shifted down at all potentials by the magnitude of Jsc, the short-circuit photocurrent. It is in the third quadrant of the J/V curve (see figure) where power is generated in an external load.
- <math>J=J_0 \left ( exp \left ( \frac {V} {n_0 k_BT/e} \right ) \right ) - J_{ph}\,\!</math>
The maximum power obtainable from an ideal OPV is the product of Jsc and the "open-circuit" photovoltage Voc (the voltage obtained for this device at zero current)
- <math>P_{theor} = J_{SC} * V_{OC}\,\!</math>
Real OPVs, however, generate substantially less power, which can be defined where real current/voltage products reach their maximum value:
- <math>P_{max} = J_{max} * V_{max}\,\!</math>
The power conversion efficiency is then defined:
- <math>(P_{max}/P_{solar})*FF\,\!</math>
where Psolar is the power from the illumination source (sun) and the Fill factor is
- <math>FF = P_{max}/P_{theor}\,\!</math>
Under AM 1.5 solar illumination conditions, most OPVs
- 0.4 < Voc < 0.8 volts,
- 5 mA/cm2 < Jsc < 15 mA/cm2,
- 0.4 < FF < 0.7, leading to power conversion efficiencies of 1-6%.
Organic Photovoltaic Fabrication and Test Apparatus
Steps in the Organic Photoelectric Process
Light Absorption (exciton formation)
Suppose you have crystalline silicon that absorbs a photon from the solar spectrum, which leads to an excited state. The exciton binding energy is so small that the electron and hole can separate and thus, a current can be produced. However, in an organic material, the absorption of a photon creates a strongly bound exciton. Remember that an exciton is a neutral species. When the electron and the hole are next to one another, the charge is 0 since 1+ + 1- = 0. A current cannot be generated from an exciton. This is why crystalline silicon can perfectly lead to an absorption to the generation of free carriers also known as the band-to-band process. Suppose an exciton is formed on a polythiophene chain. To generate current the exciton must disassociate, meaning the electron and the hole in the exciton must separate, move away from one another, and generate current.
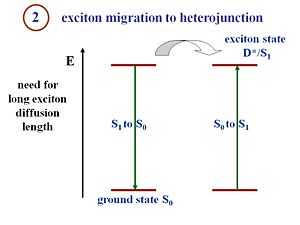
Exciton Migration
Pi- conjugated materials have large binding energies, which is the key difference between an organic solar cell and a crystalline silicon or amorphous silicon solar cell. Excitons must be formed and disassociated to generate a current. The structure of the organic cell includes a component that corresponds to an electron donor and a component that corresponds to an electron acceptor. In order to dissociate, the excitons must reach the interface between those two components.
Excitons travel a certain distance during their lifetime. This distance is called the exciton diffusion length, and is a critical component in device efficiency. To produce current, an exciton must be able to diffuse and reach the interface between the donor and acceptor components during its lifetime. If the interface is 20 nanometers from where the exciton is generated, but during its lifetime the exciton only diffuses 5 nanometers, no current will be produced. If a system has large diffusion lengths but the migration of the exciton takes too long, then the distance between where the exciton is generated and the interface must be reduced, and will result in thinner films. However, thinner films absorb fewer photons. Organic cell engineers must factor in exciton diffusion lengths, exciton lifetimes, and film thickness to design efficient cells. The film must be thick enough to absorb a reasonable amount of photons, but also be thin enough so that the generated excitons can reach the interface efficiently.
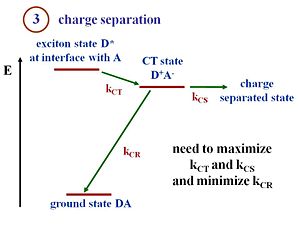
Charge Separation (exciton dissociation)
This diagram shows the events that occur when an exciton reaches the interface between the donor component (D) and the acceptor component (A).
At the interface there are donor molecules or donor polymer chains next to the acceptor molecules or acceptor polymer chains. The lowest energy state is the ground state of the two components.
Suppose that the exciton is formed within the donor. This exciton will be labeled D*, an excited state in the donor. The exciton reaches the interface. There is a state and energy there that corresponds to the exciton energy above the ground state. The exciton transfers its electron to the acceptor, forming a hole on the donor component, and creating the charge transfer state (D+ A-). The + and – charges move away from one another, forming a charge separated state.
To create current, charge transfer (CT) and charge separation (CS) both need to occur. It is possible to have a charge transfer state where the + and the – remain close to each other. However, in order to generate electrical current a full separation of the + and the – is needed. At the CT state, when the hole and electron are next to each other, there is a chance that they will recombine and lead to the ground state. This recombination represents a loss of efficiency in the charge separation process, and is the main reason for low efficiencies of OPVs.
Efficient cells will maximize the rate of charge transfer (kct) and charge separation (kcs) and minimize the rate of charge recombination (kcr). With those rates, you can also evaluate the Marcus Theory expressions.
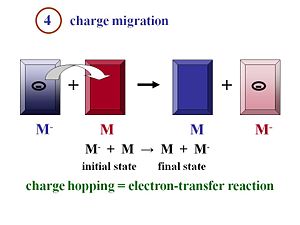
Charge Migration
Inorganic solar cells can be very efficient because a single inorganic material can be used with an exciton binding energy that is negligible at room temperature. The physics of organic solar cells is very different because the exciton is strongly bound. You need to have an acceptor and donor component with as much interface as possible between them in order that the formed excitons can quickly reach the interface to disassociate.
Once the excitons have disassociated into two separated species, the electron and the hole, they must move efficiently toward the electrodes. Remember that the more you can order your material, in general, the better the mobility will be. The faster the electrons and the holes can move away from each other, the more efficient their separation will be.
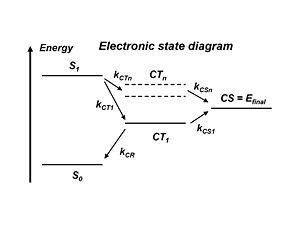
Electronic Energy Levels
The literature refers to the exciton states and the charge transfer state which is when the donor + and the acceptor – are right next to each other. But the electron and the hole still feel each other rather strongly. This charge transfer state CT1 is the lowest in energy precisely because the electron and the hole are strongly bound together. Remember, the stronger the attraction between the + and – charges, the lower the energy state. So the lowest energy charge transfer state has the strongest coulomb attraction between the hole and electron. To separate the charges, energy must be applied and a higher energy state must be acquired. In the literature there are many models that demonstrate how this can be achieved. But it would be much simpler to go directly from S1 to higher lying charge states CTn and then to CS=Efinal rather then going all the way down to CT1 and then to the final state.
With atomic energy levels, the higher the energy level of a state, the farther away the electron is from the nucleus. Compare this with CT: the higher the energy of a charge transfer state, the lesser the attraction is between the electron and the hole, and the further away the two species are from one another. So if a higher lying charge transfer state is achieved, that state will be more diffused and the wave functions will be more delocalized. Alternatively, if instead of the exciton occurring at the interface and the charges being right next to each other, if the plus is further from the minus because the wave functions are more delocalized, it will be easier for the plus and minus to move away from one another.
| Previous Topic | Return to OPV Menu | Next Topic |