Difference between revisions of "Organic/Organic Heterojunctions in OLEDs"
Cmditradmin (talk | contribs) |
|||
| Line 1: | Line 1: | ||
<table id="toc" style="width: 100%"> | <table id="toc" style="width: 100%"> | ||
<tr> | <tr> | ||
<td style="text-align: left; width: 33%">[[The | <td style="text-align: left; width: 33%">[[The First OLEDs|Previous Topic]]</td> | ||
<td style="text-align: center; width: 33%">[[Main_Page#Organic_Light_Emitting_Diodes|Return to OLED Menu]]</td> | <td style="text-align: center; width: 33%">[[Main_Page#Organic_Light_Emitting_Diodes|Return to OLED Menu]]</td> | ||
<td style="text-align: right; width: 33%">[[OLED Charge Mobilities|Next Topic]]</td> | <td style="text-align: right; width: 33%">[[OLED Charge Mobilities|Next Topic]]</td> | ||
Revision as of 13:37, 25 June 2009
| Previous Topic | Return to OLED Menu | Next Topic |
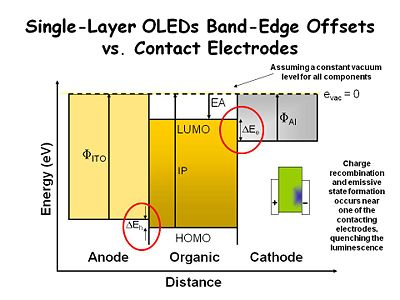
Single-Layer OLEDs Band-Edge Offsets vs. Contact Electrodes
This simplistic energy diagram shows what happens to an organic film that is sandwiched between two contacting electrodes. One side is indium tin oxide (ITO) and the other is aluminum. We are making the assumption that there is constant vacuum level for all components but this is not precisely correct. The ITO has a work function represented by the vertical arrow. For clean ITO this is typically 4.7 - 4.8ev. For dirty ITO it can be a low as 4- 4.2ev. The work function for AL is about 4.1ev. We need a work function difference between the layers because we want the aluminum to inject electrons into the layer while the ITO layer inject holes into the layer. This difference ultimately helps dictate the voltage that the device turns on.
There is an energy offset which the ionization potential of the organic layer, the energy required to take an electron out to vacuum. This something that can be measured with an UV photoelectron spectrometer. We typically do not get a good energy match between the ITO bottom contact and the organic layer. There is small energy barrier that must be overcome in order to inject holes into the organic layer. If is small it’s not a big problem.
By the same token the electron affinity EA is the distance between the vacuum level to the lumo state, that is the energy required to acquire an electron. Cathode materials have a bigger offset between the work function of the electrode and the electron affinity level. This is the energy barrier for injecting electrons into the organic layer. In an electrochemical system with a concentrated electrolyte solution the same situation occurs but it can be overcome by setting up an electrical double layer at the interface. The steep potential gradient at the interface makes it possible to get an electron injection regardless of the electrode work function. However in condensed phase environments, which have very low dielectric constants and no added ionic species in them, these energy levels are significant. Ultimately we have to go to high voltages and then tunnel charges through the energy barriers or do thermionic emission to lower drive voltages.
In the early experiments with single crystal anthracene the charge does not move at the same rate. In this case the positive charge electrode which injects holes created a charge carrier that moves faster than the electron carrier. As a result most of the recombination occurs closer to the negative electrode. That is critical problem for display devices because the metal or metal like electrodes tend to quench the emissive states before they can give off their light. They act as an energy sink. This minimized the efficiency of the devices no matter how hard they were driven. That was the state of the art before the two layer OLED were developed.
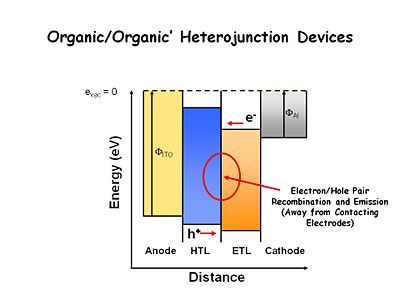
Organic/Organic’ Heterojunction Devices
This is the ubiquitous way of building a light emitting thin film system. They all involve at least one heterojunction. The transport layer (ETL) is designed to be easy to oxidize typically using bis tri aral amines. The electron transport layer is somewhat easier to reduce that the hole transport layer (HTL) and more difficult to oxidize than the hole transport layer. In electrochemical vernacular we want to find chemicals that are easy to oxidize and form stable cation radicals, and other side chemicals that are easier to reduce and form stable radical anions. This results in an energy offset between the two organic layers so that electrons once injected into the ETL layer have a large energy barrier to surmount in order to move into the HTL. The holes injected into the HTL have a large barrier to move into ETL layer. As a consequence, at low applied fields the holes and electrons build up at or near the interface. That is the site of recombination and this keeps the process away from the surface of the contacting electrodes. This was a critically important advancement.
| Previous Topic | Return to OLED Menu | Next Topic |