Solar Technologies
Return to OPV Menu | Next Topic
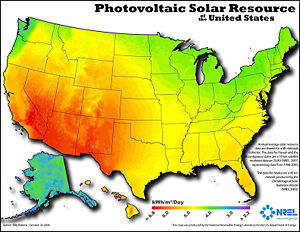
Although solar energy is a very small component of the overall sources of energy, the use of solar energy is growing significantly. But an exponential increase of the production capacity of the solar energy is still needed in order to satisfy the needs of energy.
Utilization of Solar Energy
A 42% annual growth rate of cumulative installed capacity has been fairly steady in recent years and been accompanied by a steady decrease of the price of solar modules (per watt), from nearly $100 in 1976 down to an average of $4. That trend is often referred to as the learning curve. In year 2005, for the first time in history a total of over 1 GW of power capacity was added, increasing the cumulative installed capacity by 42% reaching a value of 3.7 GW in the IEA PVPS countries (90% of the worldwide production). The greatest proportion was installed by Germany and Japan alone (85%).
Solar energy can be used even for a household. Solar cells may be on the top of the roof and solar energy can be collected for use in a household. BOS (balance of system) cost is larger for off-grid applications because they require a storage device. That solar energy could also be distributed back into the electrical grid when there is an overproduction with respect to your own needs. This distributed system is useful because every house can collect the energy not only for themselves but they can also transfer the source back on the electrical grid. You can also centralize power stations that would be grid connected and would work as normal electrical power stations.
Generations of Solar Cells
- First Generation Solar Cells
The solar cells that you have on the market are made out of crystalline silicon, multcrystalline ribbon, or ingot, are also known as the "first generation solar cells". Right now the majority of the modules of the cells come from crystalline silicon. The supply of silicon is starting to tighten. Other elements such as indium that is the most common transparent conductor in the form of ITO is also increasing in demand. Amorphous silicon is also being used but less efficient. Then you have thin films like amorphous silicon which is much less costly in terms of its application. The power conversion efficiency for silicon is about 20%. (Pmax/Psun) Because they are made from crystalline silicon they are structurally rigid which makes them hared to process and distribute.
See DOE report on solar energy research[1]
- Second Generation Solar Cells
Other films like CIGS which is copper indium gallium selenide or cadmium teluride or III-V for instance gallium indium are the group three elements. Those thin films are referred to as the 2nd generation solar cells.
- Third Generation Solar Cells
With organics and the newer components like the hybrid organic or inorganic cells, you are talking about the 3rd generation solar cells.
Solar Potential
A sun power generator is used to test the efficiency of organic solar cells. The sun power generator refers to the air mass (AM) 1.5 = Air Mass which also corresponds to the incident power of 100 mW/cm^2 or 1000 W/m. Questions like “What is the efficiency of your solar cell in terms of transforming that amount of power per cm^2 into electrical power?” come up often. This is referred to as one sun.
This green curve shows solar spectral distribution for one sun (the y-axis units on the right) for those standardized conditions and the black curve (y-axis on the left) represents the total current density that you have when you integrate from a wavelength of 0. The maximum current density can be calculated by counting all the photons starting from 0 wavelength on the high energy side. This the maximum current density that can be obtained if the power efficiency were to be 100%, or in other words, if for every photon that comes, one electron enters the electrical circuit.
Cost Considerations
The major driver of new technologies is not only the lack of supply. If an alternative solution for sources of energy that is less expensive than fuel or electricity is found, then everyone will use it as long as the technology is available. However, as of now, that is not the case. The cost of electricity from coal fired thermal plants is about 4 cents a kilowatt hour while silicon solar runs about 25- 35 cents a watt. The price for alternative solutions such has solar cells is still pretty high. For instance, Allen Heeger[2] has installed a solar cell roof on his house in Santa Barbara but it will take about 7-8 years before it becomes profitable. However, that is still better than losing money from electricity costs every year for the duration or lifetime of the house. Metrics: What is most important in terms of the overall production is power conversion efficiency of single cell. With respect to the given input power of the sun, what is the electrical output power that the solar cell can produce? In our case, we will refer to this power conversion efficiency
An economic example: with 5 hours of peak sun per day, 10% conversion efficiency and 10 m2 (1 kW capacity), 5kWh/day, 150 kWh per month, 1,800 kWh per year would product $600 of electricity per year if $0.3 per kWh. The cost of a 1kW capacity system, $7/W, $7,000.
Carbon footprint
The Problem: How much coal must you burn (and how much CO2 will you produce) to generate enough electrical energy to charge up your iPod/cellphone?
A typical lithium ion battery generates about 3.7 volts
The approximate half-cell reactions and overall reaction: xLi+ + xe- ⇄ Liºx(graphite) Eº = -3.05 volts Li1-xMn2O4(s) + xLi++ xe- ⇄ LiMn2O4(s) Eº = +0.65 volts Liºx(graphite) + Li1-xMn2O4(s) ⇄ LiMn2O4(s) Eºcell = +3.7 volts
You can computer the maximum amount of work Assumption #1: An average cell phone/iPod battery contains about 7 grams (1 mole of Li) Work that can be done by the battery (during discharge/energy production): wmax = -(coulombs) · Ecell ≈ (1 mole Li) · (96,500 coulombs/mole)·(3.7 volts) ≈ -357 kJoules (in fact you won’t get this much, you might get ca. 1/2 of this amount, since you won’t use all of the available Li° and you are limited by rates of electron transfer at both anode and cathode Energy output ≈ -178.5 kJoules
Efficiency
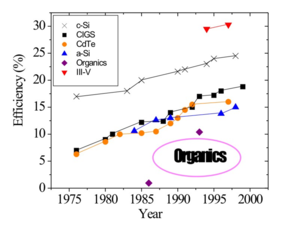
The record in terms of power efficiency for a single cells on the order of 25%. If cells are stacked up with one on top of the other, the power efficiency can reach up to 40%. The material that can produce the best performance and has the highest power efficiency is the III-V. Crystal and silicon has an efficiency of 25% for a single cell. The organics that have started 20 years ago with the work of Xing Tai at Kodak are going up. For polymers, the record efficiency is on the order of 6%. In the case of the Gretal cells that are hybrid organic, inorganic, can have efficiencies of 11-12%. Usually there is too much emphasis on efficiency of the cell because that is certainly not the only parameter that needs to be taken into account in the engineering of the cell and in using it efficiently for the electrical grid. Many of these cells that produce a very high efficiency are also extremely small. Then when solar cells are scaled up to the sizes that are needed to cover a roof the scaling up will lead to issues that will lower the efficiency. So when someone gives you a new record efficiency always pay attention to what is the scale or the area of the cells they are using.
Weight and Flexibility
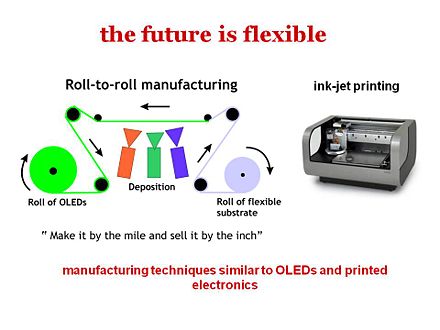
Another reason many people have interests in organic solar cells is that flexible modules can be made. For example instead of separate panels, extremely thin conformable films can be used to cover the roof and the solar cells won’t make any visual impact on your roof. Also for many other applications, weight is an issue. For soldier in operations a very significant part of the weight that must be carried is from due to batteries and therefore, being able to have portable power that would be light weight would make a big difference. All these aspects stir great interest in making flexible organic cells.
Prototype Fabrication and Testing
Organic photovoltaic devices are typically built with either the technique of vacuum deposition or spin coating. Either system allows the progressive build up of very thin uniform layers of chemicals and metallics. In spin coating the test surface is coated with a chemical and then spun at high speeds in a centrifuge so that excess material thins out. Vacuum deposition allows building up of layers one atom at a time and permits very precise control over the surface characteristics. Prototype cells are then tested with and "artificial sun" lamp that is calibrated for power.
<swf width="800" height="600">http://depts.washington.edu/cmditr/media/opvfab.swf</swf>
http://en.wikipedia.org/wiki/Spin_coating