Difference between revisions of "Organic Heterojunctions in Solar Cells"
(New page: These are the levels or the states. It is important to understand the differences between the levels that refer to one electron level picture and states that refer to the whole molecule ...) |
Cmditradmin (talk | contribs) |
||
| Line 1: | Line 1: | ||
[[Main_Page#Organic Solar Cells|Return to OPV Menu]] | | |||
[[Physics of Solar Cells|Next Topic]] | |||
[[Image:Organicheterojunctions.JPG|thumb|300px|]] | |||
== Energy levels in heterojunctions == | |||
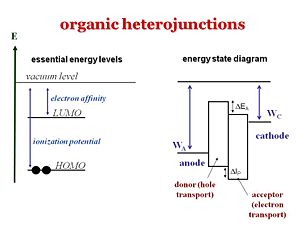
These are the levels or the states. It is important to understand the differences between the levels that refer to one electron level picture and states that refer to the whole molecule or system. The ionization potential of a molecule can be approximated as the HOMO, the energy or the inverse of the energy of the HOMO level. The electron affinity can be related to the energy of the LUMO level. But one must not forget that the ionization potential and electron affinity are characteristics of the whole molecule. The energy state diagram refer to states, the difference in energy between the ground state and the ionized state of the whole molecule. Where as HOMOS and LUMOS refer to electron levels. When organic solar cells are discussed, we must pay attention to the electron affinities and ionization potentials of the donor and acceptor components. An energy diagram includes the WA work function of the anode, Wc the work function of the cathode; similar to the diagram for light emitting diodes. The ionization potential of the donor is the difference between the neutral molecule and the energy it takes to take away an electron from that molecule. HOMO and LUMO levels are often seen in the literature but these are only approximations. We need to take into account are the ionization potentials and the electron affinities of both the donor and the acceptor. In order for this concept of the donor and acceptor to be valid, the ionization potential of the donor should be lower than that of the acceptor because the donor is the species that most easily gives away an electron. In this diagram the ionization energy of the donor is the distance between to top of the donor band and the vacuum level band above. Conversely, the electron affinity of the acceptor should be larger than the electron affinity of the donor. | |||
Consider a system with two compounds, A and B. If compound B has lower Lumo and a higher Homo than compound A you would not expect separation of charges. If there is an excess electron it will go the lowest Lumo which is compound B. If there is an excess hole it will go to the highest homo which is also compound B. The excess electron and the hole will want to be in this compound B so there won’t be any charge separation; it will not be favored. | |||
A favored situation is shown where the LUMO of the donor is higher than the LUMO of the acceptor, and the HOMO of the donor is higher than that of the homo of the acceptor. This is a situation where charge separation might be possible but it doesn’t guarantee that it will happen. It is a necessary but it not sufficient condition. For charge separation to occur the combination of these differences in electron affinities and ionization potentials must have the ability to overcome the exciton binding energy. For example in the case of ppv and cyano ppv the levels that are aligned like this but still there is no charge separation. There is energy transfer but no charge separation. | |||
Consider these structures in the absence of any external field. There are two semi conductors, with a work function of the anode, and the work function of the cathode. The work function refers to the Fermi level that is the chemical potential of the two electrodes. When a system is in equilibrium, the system tends to get the chemical potentials to be the same across the entire system. In order to reach an equilibrium, the system will tend to have some charge redistribution that will increase chemical potential on the anode side and decrease the potential energy on the cathode side so that the two chemical potentials can try to align. | |||
Since the chemical potential of the anode and the cathode are different, there will be some charge redistribution to increase the energy on the anode side and decrease the chemical potential on the cathode side. This will create a bias of the levels within the organic semiconductor or insulators, which is called a built in potential or built in electrical field. In the physics literature, a description of what is happening can be found in the context of metal insulators and metal structures. This is what allows you to understand the fact that if there is an elimination and an electron hole pair is formed at and at the interface the electron will travel to the acceptor side a hole will be left on the donor side. This built in potential makes the electron drift towards the cathode and the hole drift towards the anode. Without that built in field, there will be no incentive for the electron to drift one way or the other; the electron and hole would diffuse. However, the built in potential gives a direction for the motion of the separated charge carriers. | |||
== Excitonic Solar Cells == | |||
[[Image:Hjsteps.JPG|thumb|300px|]] | |||
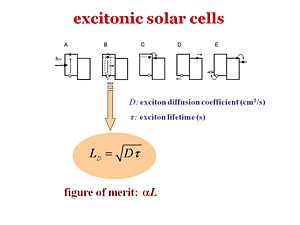
There are five different steps in excitonic solar cells. These 5 steps should also be valid for the organic light emitting diodes-- the fifth step would be the out coupling. The slide shows the energy scheme. The first step A requires a photon absorption. What is most favorable is to have a material that absorbs as intensely and broadly as possible across the solar spectrum. Then the excitons are formed in Step B. This whole process occurs in excitonic solar cells. On the other hand, in organic semi conductors, this exciton part is not dealt with. Actually the very first organic solar cells that has been described in the literature in 1959 by Martin Pope and his workers used a single component called anthracene. The number of charges that were generated was extremely low because when the exciton was formed, it remained bound. Thus, not many charges were separated and little current was generated which leads to a poor efficiency. That is particularly why ChingTang made a major advance using heterojunctions that consists of a donor and an acceptor. At step B, the exciton diffuses. LD refers to the diffusion length which is directly proportional to the diffusion coefficient D times the lifetime T of the exciton. The diffusion coefficient D represents how fast the exciton can diffuse within the material or how fast the exciton can hop from molecule to molecule, or from chain segment to chain segment. The larger the diffusion coefficient, the longer the lifetime, which means the further the exciton can go before it decays back to the ground state. It is better if the exciton has a long diffusion length LD because it allows the exciton to have a higher chance of reaching the interface with the acceptor within its lifetime. If this process does not occur, no charge will generate and the exciton will decay back to the ground state. This decay will either produce heat, vibrations, or release the photons it once absorbed-- also called photoluminescence. But photoluminescence is not what you want in solar cell. As you can see, the LD=sqrt(Dt) equation is an important parameter. Alpha L is the figure of merit . It is the product of the diffusion length and the absorption coefficient or the absorbance of the material. The most efficient organic layer would be very absorbing, have a large alpha, and the excitons would have a large diffusion length so that the exciton will have a large probability of finding the interface during its lifetime. | |||
The third step C is when the disassociation, or charge separation, occurs at the interface. Once the electron and the hole have separated, the built in potential cause the electron to drift toward the cathode and the hole to drift toward the anode. If there was no built in potential, the electron and the hole will simply diffuse without any directionality to their motion. | |||
In the last step E, the electron and the hole will be collected efficiently at their respective electrodes, generate a current, and enter the external circuit | |||
== Geometries of excitonic solar cells == | |||
[[Image:Opvgeometry.JPG|thumb|300px|]] | |||
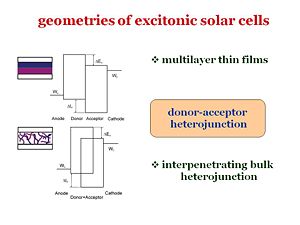
Recently, there were steady improvements in efficiencies of organic solar cells. Whether it is based on multilayer thin film geometry or intepenerating polymer blend, the efficient light harvesting relies on donor-acceptor heterojunction in most cases. Therefore, it is our purpose to establish the model that characterizes the photoelectrical response of the cellls based on those donor-acceptor heterojunctions. | |||
These are the two major geometries that have been considered. For example, from Chin Tang’s paper in 1986, one of the geometries included a bi-layer: it had an anode, cathode, donor component, and an acceptor component. The idea of Chin Tang is to move away from a homojunction that uses a single component, and go to a two component system with a donor and an acceptor. However, an issue with this type of system is that the diffusion length must be quite large in order for the exciton to reach the interface and disassociate. It is possible to make the layer thinner so that the distance to the interface is shorter, but in doing so, the layer will be less absorbing and fewer photons will be absorbed. So the idea in the mid 90’s, from the group of Allen Heegger in Santa Barbara, was to build a bulk heterojunction. Bulk heterojunctions are made by creating interpenetrating networks of the donor and the acceptor components. With the two components interpenetrating one another, an exciton will never be far from the interface. In addition, with respect to the volume, the surface represented by the interface will be larger. The interfacial area will be large and the diffusion length of the excitons can be short. This puts many constraints on how to manage the morphology of the interpenetrating network. This is due to the fact that once the disassociation has taken place, the electron and the hole need to have a clear path to get to the cathode and the anode respectively. If the morphology is such that the path closes somewhere along the way, the electron and the hole will be lost. Bulk heterojunctions, are described as systems of interpenetrating “fingers” of the donor component and the acceptor component sandwiched between the anode and cathode. This system has a large interfacial area, the interface is not far from where the exciton is generated, and once the exciton disassociates, the electron and the hole will have a clear path to their respective electrodes. | |||
== Bulk heterojunctions == | |||
[[Image:Bulkhj.JPG|thumb|300px|]] | |||
You are often are limited by the diffusion length of the exciton. All these factors contribute to why people have spent so much time trying to design efficient bulk heterojunctions. The morphology must be controlled so that the electron and the hole can always go back to the cathode and anode respectively. Also there should not be many places where the hole and the electron see each other and can recombine. | |||
== Pioneers in organic photovoltaics == | |||
[[Image:Opvpioneers.JPG|thumb|300px|]] | |||
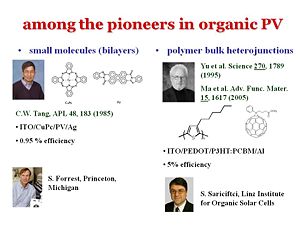
There are a few people who have advanced the field. Chin Tang used to work for the Kodak research center but moved to become a full professor at University of Rochester in 1986. There have also been major advances from Steve Forrest who was at Princeton for quite some time and had recently transferred to his alma meter as VP for research at Michigan. In terms of polymers, a major step forward in understanding was made when Serdar Sariciftci was working as a research scientist in Allen Heeger’s groups in the early 90’s. They discovered an ultra-fast charge separation at the interface between a PPV derivative and C60. The charge separation occurred within about 30 – 40 femptoseconds. Since the charge separation is so much faster than any other process, it leads to a charge separation of unity. That is why there has been so much work on phenyline, vinylines with c60 or c60 derivatives as well as more recently on polythiophenes or derivatives thereof and C60 and derivates thereof. | |||
Revision as of 12:51, 5 May 2009
Return to OPV Menu | Next Topic
Energy levels in heterojunctions
These are the levels or the states. It is important to understand the differences between the levels that refer to one electron level picture and states that refer to the whole molecule or system. The ionization potential of a molecule can be approximated as the HOMO, the energy or the inverse of the energy of the HOMO level. The electron affinity can be related to the energy of the LUMO level. But one must not forget that the ionization potential and electron affinity are characteristics of the whole molecule. The energy state diagram refer to states, the difference in energy between the ground state and the ionized state of the whole molecule. Where as HOMOS and LUMOS refer to electron levels. When organic solar cells are discussed, we must pay attention to the electron affinities and ionization potentials of the donor and acceptor components. An energy diagram includes the WA work function of the anode, Wc the work function of the cathode; similar to the diagram for light emitting diodes. The ionization potential of the donor is the difference between the neutral molecule and the energy it takes to take away an electron from that molecule. HOMO and LUMO levels are often seen in the literature but these are only approximations. We need to take into account are the ionization potentials and the electron affinities of both the donor and the acceptor. In order for this concept of the donor and acceptor to be valid, the ionization potential of the donor should be lower than that of the acceptor because the donor is the species that most easily gives away an electron. In this diagram the ionization energy of the donor is the distance between to top of the donor band and the vacuum level band above. Conversely, the electron affinity of the acceptor should be larger than the electron affinity of the donor.
Consider a system with two compounds, A and B. If compound B has lower Lumo and a higher Homo than compound A you would not expect separation of charges. If there is an excess electron it will go the lowest Lumo which is compound B. If there is an excess hole it will go to the highest homo which is also compound B. The excess electron and the hole will want to be in this compound B so there won’t be any charge separation; it will not be favored.
A favored situation is shown where the LUMO of the donor is higher than the LUMO of the acceptor, and the HOMO of the donor is higher than that of the homo of the acceptor. This is a situation where charge separation might be possible but it doesn’t guarantee that it will happen. It is a necessary but it not sufficient condition. For charge separation to occur the combination of these differences in electron affinities and ionization potentials must have the ability to overcome the exciton binding energy. For example in the case of ppv and cyano ppv the levels that are aligned like this but still there is no charge separation. There is energy transfer but no charge separation.
Consider these structures in the absence of any external field. There are two semi conductors, with a work function of the anode, and the work function of the cathode. The work function refers to the Fermi level that is the chemical potential of the two electrodes. When a system is in equilibrium, the system tends to get the chemical potentials to be the same across the entire system. In order to reach an equilibrium, the system will tend to have some charge redistribution that will increase chemical potential on the anode side and decrease the potential energy on the cathode side so that the two chemical potentials can try to align. Since the chemical potential of the anode and the cathode are different, there will be some charge redistribution to increase the energy on the anode side and decrease the chemical potential on the cathode side. This will create a bias of the levels within the organic semiconductor or insulators, which is called a built in potential or built in electrical field. In the physics literature, a description of what is happening can be found in the context of metal insulators and metal structures. This is what allows you to understand the fact that if there is an elimination and an electron hole pair is formed at and at the interface the electron will travel to the acceptor side a hole will be left on the donor side. This built in potential makes the electron drift towards the cathode and the hole drift towards the anode. Without that built in field, there will be no incentive for the electron to drift one way or the other; the electron and hole would diffuse. However, the built in potential gives a direction for the motion of the separated charge carriers.
Excitonic Solar Cells
There are five different steps in excitonic solar cells. These 5 steps should also be valid for the organic light emitting diodes-- the fifth step would be the out coupling. The slide shows the energy scheme. The first step A requires a photon absorption. What is most favorable is to have a material that absorbs as intensely and broadly as possible across the solar spectrum. Then the excitons are formed in Step B. This whole process occurs in excitonic solar cells. On the other hand, in organic semi conductors, this exciton part is not dealt with. Actually the very first organic solar cells that has been described in the literature in 1959 by Martin Pope and his workers used a single component called anthracene. The number of charges that were generated was extremely low because when the exciton was formed, it remained bound. Thus, not many charges were separated and little current was generated which leads to a poor efficiency. That is particularly why ChingTang made a major advance using heterojunctions that consists of a donor and an acceptor. At step B, the exciton diffuses. LD refers to the diffusion length which is directly proportional to the diffusion coefficient D times the lifetime T of the exciton. The diffusion coefficient D represents how fast the exciton can diffuse within the material or how fast the exciton can hop from molecule to molecule, or from chain segment to chain segment. The larger the diffusion coefficient, the longer the lifetime, which means the further the exciton can go before it decays back to the ground state. It is better if the exciton has a long diffusion length LD because it allows the exciton to have a higher chance of reaching the interface with the acceptor within its lifetime. If this process does not occur, no charge will generate and the exciton will decay back to the ground state. This decay will either produce heat, vibrations, or release the photons it once absorbed-- also called photoluminescence. But photoluminescence is not what you want in solar cell. As you can see, the LD=sqrt(Dt) equation is an important parameter. Alpha L is the figure of merit . It is the product of the diffusion length and the absorption coefficient or the absorbance of the material. The most efficient organic layer would be very absorbing, have a large alpha, and the excitons would have a large diffusion length so that the exciton will have a large probability of finding the interface during its lifetime. The third step C is when the disassociation, or charge separation, occurs at the interface. Once the electron and the hole have separated, the built in potential cause the electron to drift toward the cathode and the hole to drift toward the anode. If there was no built in potential, the electron and the hole will simply diffuse without any directionality to their motion. In the last step E, the electron and the hole will be collected efficiently at their respective electrodes, generate a current, and enter the external circuit
Geometries of excitonic solar cells
Recently, there were steady improvements in efficiencies of organic solar cells. Whether it is based on multilayer thin film geometry or intepenerating polymer blend, the efficient light harvesting relies on donor-acceptor heterojunction in most cases. Therefore, it is our purpose to establish the model that characterizes the photoelectrical response of the cellls based on those donor-acceptor heterojunctions. These are the two major geometries that have been considered. For example, from Chin Tang’s paper in 1986, one of the geometries included a bi-layer: it had an anode, cathode, donor component, and an acceptor component. The idea of Chin Tang is to move away from a homojunction that uses a single component, and go to a two component system with a donor and an acceptor. However, an issue with this type of system is that the diffusion length must be quite large in order for the exciton to reach the interface and disassociate. It is possible to make the layer thinner so that the distance to the interface is shorter, but in doing so, the layer will be less absorbing and fewer photons will be absorbed. So the idea in the mid 90’s, from the group of Allen Heegger in Santa Barbara, was to build a bulk heterojunction. Bulk heterojunctions are made by creating interpenetrating networks of the donor and the acceptor components. With the two components interpenetrating one another, an exciton will never be far from the interface. In addition, with respect to the volume, the surface represented by the interface will be larger. The interfacial area will be large and the diffusion length of the excitons can be short. This puts many constraints on how to manage the morphology of the interpenetrating network. This is due to the fact that once the disassociation has taken place, the electron and the hole need to have a clear path to get to the cathode and the anode respectively. If the morphology is such that the path closes somewhere along the way, the electron and the hole will be lost. Bulk heterojunctions, are described as systems of interpenetrating “fingers” of the donor component and the acceptor component sandwiched between the anode and cathode. This system has a large interfacial area, the interface is not far from where the exciton is generated, and once the exciton disassociates, the electron and the hole will have a clear path to their respective electrodes.
Bulk heterojunctions
You are often are limited by the diffusion length of the exciton. All these factors contribute to why people have spent so much time trying to design efficient bulk heterojunctions. The morphology must be controlled so that the electron and the hole can always go back to the cathode and anode respectively. Also there should not be many places where the hole and the electron see each other and can recombine.
Pioneers in organic photovoltaics
There are a few people who have advanced the field. Chin Tang used to work for the Kodak research center but moved to become a full professor at University of Rochester in 1986. There have also been major advances from Steve Forrest who was at Princeton for quite some time and had recently transferred to his alma meter as VP for research at Michigan. In terms of polymers, a major step forward in understanding was made when Serdar Sariciftci was working as a research scientist in Allen Heeger’s groups in the early 90’s. They discovered an ultra-fast charge separation at the interface between a PPV derivative and C60. The charge separation occurred within about 30 – 40 femptoseconds. Since the charge separation is so much faster than any other process, it leads to a charge separation of unity. That is why there has been so much work on phenyline, vinylines with c60 or c60 derivatives as well as more recently on polythiophenes or derivatives thereof and C60 and derivates thereof.