Difference between revisions of "Electronic Coupling Between Orbitals"
Cmditradmin (talk | contribs) |
Cmditradmin (talk | contribs) |
||
| Line 10: | Line 10: | ||
In the Hückel approximation this energy is the same for all carbon p-orbitals that are adjacent to on another and is called Β. | In the Hückel approximation this energy is the same for all carbon p-orbitals that are adjacent to on another and is called Β. | ||
<br clear='all'> | |||
[[Image:Beta-nonadjacent.png|thumb|200px|Non- Adjacent p orbitals do not interact]] | [[Image:Beta-nonadjacent.png|thumb|200px|Non- Adjacent p orbitals do not interact]] | ||
For atoms that are not adjacent to one another Β is taken to be zero. Thus in the Hückel approximation there is no interaction between nonadjacent atoms. | |||
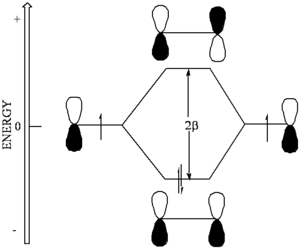
[[Image:Beta-energydiagram.png|thumb|300px|]] | |||
Through this coupling of atomic orbitals, molecular orbitals can formed by a constructive and destructive combination of the atomic orbitals. | |||
Revision as of 11:47, 19 May 2009
Return to Molecular Orbitals Menu | Next Topic
We will discuss the electronic structure of π-conjugated organic molecules at various levels of complexity. π-conjugated molecules have a sigma electron framework and π electron framework.
Here we start at the simplest level that is basically derived from a Hückel Molecular Orbital approach in order to understand conjugated systems.
In this model, orbitals that are on atoms that are directly σ-bonded to one another and whose p-orbitals are in a plane can interact. This interaction is called the "electronic coupling" between the orbitals and has units of energy.
In the Hückel approximation this energy is the same for all carbon p-orbitals that are adjacent to on another and is called Β.
For atoms that are not adjacent to one another Β is taken to be zero. Thus in the Hückel approximation there is no interaction between nonadjacent atoms.
Through this coupling of atomic orbitals, molecular orbitals can formed by a constructive and destructive combination of the atomic orbitals.