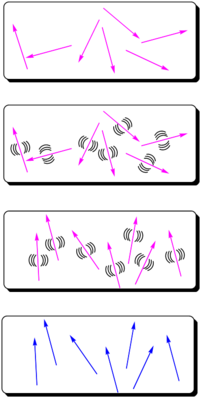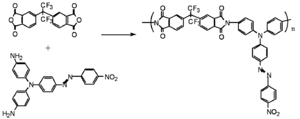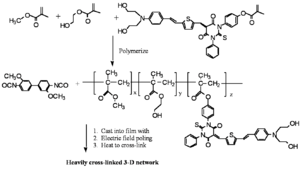Difference between revisions of "Second-order NLO Materials"
Cmditradmin (talk | contribs) |
Cmditradmin (talk | contribs) |
||
| Line 231: | Line 231: | ||
Dield-Alder Crosslinked Systems allows you to produce material that is a highly soluble polymer for purification and film casting (because of the protected maleimide, but then can be heated, deprotected, poled and locked into place with cross linking. | |||
These systems have a high R33 are Thermally stable: ~80% of the original value can be retained at 70 °C for more than 250 hrs. | |||
see Jen et al 2002 <ref>Jen et al., Adv. Mater., 14, 1763 (2002)</ref> | see Jen et al 2002 <ref>Jen et al., Adv. Mater., 14, 1763 (2002)</ref> | ||
Revision as of 11:37, 9 July 2009
| Previous Topic | Return to Second-order Processes, Materials & Characterization Menu | Next Topic |
Linear Electro-optic Effect
The polarization density can be written as a Taylor series
- <math>P_{ind} = \chi^{(1)} E_1 + 1/2 \chi^(2) E_1 E_2 + 1/6 \chi^(3) E_1 E_2 E_3 + ... \,\!</math>
Index of refraction is related to susceptibility (and therefore ultimately back to polarizability) by:
- <math>n^2 = 1 + 4\pi \chi\,\!</math>
Electro-optic effect Consider the case where one field is E1cos[ωt] and second field is a static DC electric field (or very low frequency DC field) of magnitude E2. The second-order term of the induced polarization simply becomes:
- <math>\chi^{(2)} E_1 E_2 cos[\omega t]\,\!</math>
and therefore:
- <math>P = ( \chi^{(1)} + 1/2\chi^{(2)} E_2 ) E_1 [cos \omega t]\,\!</math>
Notice that the the second term χ(2) is field dependent.
Thus, when an electric field is applied to such a poled polymer, the index of refraction of the material will change. This sensitivity of the material to having its index of refraction changed by application of an external electric field is characterized by its so-called electro-optic coefficient, coefficient (r33).
For many years it had been suggested that electro-optic polymers could exhibit large r33 greatly surpassing that of a technologically important crystal, lithium niobate. It is transparent, it is inexpensive, it can be grown in large sheets, and it has an r33 of 30.5 pm/V. This is a reasonable r33 that allows you to build devices. When we build organics we have synthesize new compounds, worry about stability, worry about orientation, and how to keep them poled. But there are a significant advantage to organics.
While there are both electronic and vibrational components to the non-linearity in lithium niobate, a large component is vibrational. This puts an upper limit on the frequency with which that LiNbO3 can be modulated. If a driving field approaches the vibrational frequency then the molecules can not respond quickly to the applied field. The response trails off and there is only the electronic contribution for the non-linearity.
Organic materials are completely dominated by the electronic contributions and therefore can be modulated at very high frequencies.
It is also possible to get organics with an r33 that is much higher (for example 55 pm/V) than LiNbO3. This means that the Vpi (the voltage required for a π phase shift) can be less to get the same change in optical property.
Finally it possible to integrate electronic with optical parts right on a chip. Polymer technology is going to make it much easier to combine these materials on a chip.
In the field of silicon photonics there are waveguides built with ebeam lithography on which the polymer can be spin coated and fill the channels. Organics are amorphous whereas LiNbO3 is a single crystal material which makes it very challenging to apply to chip-scale device.
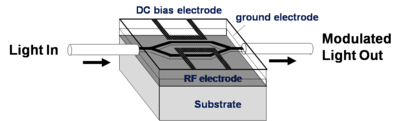
EO-Modulators
An electrode is located on either side of the light path which passes through an electro optical material. An opposite field can be applied to each path slowing the light on one side and speeding the light on the other. There are many factors that contribute to the figure of merit Vpi (the voltage that is required to shift the wave one half of a wavelength). A longer optical wavelength is better. A long interaction length gives more time for the material to respond to the field, but this means the optical material must be very clear so that optical intensity is not lost. Light intensity can be lost by absorption, diffraction and scattering. The 1.31 microns and 1.55 microns are popular telecommunication wavelengths because they are easily propagated through optical fiber. This is the typical wavelength that the modulators need to work with. Many NLO materials absorb in the 650- 750 nm range. 640 nm is about 2 eV (1ev = 1240/ wavelength nm); 1.55 microns is about .8 eV. A lot of the molecules are absorbing at about 700nm (1.7 eV). The absorption tail of a molecule can go out a long ways. At some point you have to worry about C-H bonds because of overtones due the C-H stretching frequency that occur at 3000 cm-1 (3333 nm). This means there could be some absorption at 1600 nm (1/2 of 3333 nm) due to C-H overtones. This is a problem.
Another problem is that the high concentrations of chromophores in these systems results in chromophore-chromophore interactions, inhomogeneities of the chromophore concentrations, and chromophore polymer interactions. Each of these factors leads to a variation of the refractive index which can lead to the scattering of light at some level. If the interaction length of the light in the Mach Zehnder devices needs to be long then it is important to keep the scattering in the organic component to a minimum.
It is important to have a high index of refraction and high electro-optic coefficient. Index of refraction relates to dielectric constant which relates to susceptibility, which relates to polarizability (α), which relates to the transition dipole moment within the context of a two level model. Change of dipole of moment is the state dipole moment for the excited state minus the state dipole moment for the ground state. Transition dipole moment is an integral of the complex conjugate of the excited state wavefunction, the transition dipole moment operator (which scales like R) and the ground state wave function Dτ. The transition dipole moment relates to the oscillator strength (the area under the absorption curve) which is related to the extinction coefficient. Organic dyes that are used in second order NLO systems are materials that have a high refractive index and strong low energy absorptions.
Poling of Chromophores in Polymers
In order to achieve a high electro-optic coefficient you start with molecules with a high first hyperpolarizability (beta), and then get those molecules oriented in the same direction. (For now we will examine orientation for high EO coefficient and will ignore the orientation required for second harmonic generation which require a different orientation)
Orientation can be achieved with crystalline materials. The Marder group was able to develop crystals of DAST. (They were working with a Japanese group at the same time who succeeded in making a crystal of the hydrate of the material due to their high local humidity. Marder’s group in California was able to make the DAST crystal due to the low local humidity. One crystal was centro symmetric and one was non-centrosymmetric.) Growing crystals is very difficult. Another group spent 10 years developing the method to grow one crystal. (Rainbow Photonics)
The most widely used method for aligning molecules is the poled polymer method. Recall that dipole moment is a vector, and first hyperpolarizability beta is a tensor. Assume that the molecule has one axis (the long axis) along which the hyperpolarizability beta dominates. If the dipole moment is aligned along the long axis then an applied electric field will cause a torque to reorient the molecules. (Boltzman will be working against this attempting to randomize the orientation) There will be a distribution of orientations but if you sum over all the vectors there will be a net orientation. This in turn aligns the hyperpolarizability tensor. In the limiting case in which the hyperpolarizability is perpendicular to the dipole moment there will be an alignment of the hyperpolarizability tensor in a plane but within that plane the tensors will be oriented at all angles. This results in a zero overall second order optical activity. Therefore we need molecules with large dipole moments that are oriented along the same axis as the hyperpolarizability tensor.
- An NLO chromophore with a dipole moment (such as paranitroaniline) and a glassy polymer are dissolved in an organic solvent and spin-coated onto a substrate. The film is then heated above or around its glass transition temperature. The glass transition temperature at which there is free motion of the main chain of the polymer.
- At this temperature, the molecules can rotate in the rubbery matrix. An intense DC electric field as high as 106 V/cm is applied and this field creates a force on the dipole moments of the chromophores, aligning them.
- Thus, the polymers and chromophores must be nonconducting to support these large fields. With the electric field still applied, the film is cooled below the Tg of the polymer, restricting the motions of the chromophores.
In this manner you can produce a material that has a second order nonlinear optical effect using molecules that have a second order nonlinear optical effect.
The resulting noncentrosymmetric structure contains the molecular units oriented on the average normal to the film (C∞v symmetry). In this state, the electric field of an optical beam propagating through the film can be maximally modified when the field is parallel to the orientated molecular units.
An amorphous polymer above its Tg should have the ability for large scale reorientations of the molecules.
The magnitude of the second-order NLO effect in a poled polymer film is related to several factors.
Using a non-interacting oriented molecular gas model, the follow equation has been derived to describe the second-order susceptibility in the direction of the poling field (χ (2)333). It is calculated:
- <math>\Chi^{(2)}_Template:333 = N F \beta_{\mu} <cos^3\theta>\,\!</math>
Where:
- <math>N\,\!</math> is the number density of the chromophores in the material
- <math>F\,\!</math> is
- <math>\beta _mu\,\!</math> is the component of beta that is aligned along the axis of the dipole moment (eg not 90degrees)
and where:
- <math><cos3 \theta> = \mu_z E_p /cKT\,\!</math>
- <math>\mu_z\,\!</math> is the dipole moment of the molecule (the larger the dipole, the more it will respond to the field)
- <math>E_p\,\!</math> = the strength of the electric field
- <math>k\,\!</math> is the Boltzman constant, T is temperature in Kelvin
- <math>c\,\!</math> is a system dependent factor equal to 5 for isotropic systems, and 1 for ideal liquid crystalline systems (Ising systems)
In liquid crystals the orientation of one molecule can affect neighboring molecules (cooperativity) <cos3θ> is the induced polar order which is an average angular orientation where theta is the angle between the poling axis and the molecular dipole moment of the chromophores. Molecules with large dipole moments will tend to repel and that interferes with poling especially when they get too close (high concentration).
This equation is valid a low field, :<math>\mu_zE_p < kT\,\!</math>.
Key Molecular Properties for Poled Polymers
- large optical nonlinearities (large χ(2) - large r33)leading to low drive voltages.
- thermal stability - high temperatures in fabrication (want to pole 150 - 200°C above Tg) and high operating temperatures –very few organic dyes are are stable above 250°C- molecules can be engineered to remove weak points.
- photochemical stability - materials undergo intensive irradiation during applications
- stable against oxidation- and reduction processes due to high electrical fields in fabrication and exposure to air (oxygen)
- minimized self aggregation - aggregation causes scattering loss and aggregation makes molecules harder to orient - mitigated by covalent linkage to polymer-backbone
- long-term orientational stability of devices- for devices that are deployed in telecommunication (underground) they should last at least 75 years.
Schematic of Poled Polymers Strategies
The poled orientation is thermodynamically unstable and therefore the orientation quickly decays in low Tg polymers such as poly(methylmethacrylate), resulting in a greatly reduced nonlinearity. The diagram show several strategies for linking chromophores to polymers so they are more constrained.
The motion of chromophores in polymer matrices might be further restricted if the chromophore is covalently attached at one or more sites to the polymer. One end of the chromophore is pinned down it may more thermally stable. This also reduces the tendency for the polymer and chromophore to separate into phases because they are essentially glued together.
Accordingly, several groups have explored covalently attached chromophores to polyimides either in the main chain or as a side chain. This approach can lead to polymers with exceptional thermal stability. Lately, Jen's group has reported a 2-step synthesis for NLO side-chain aromatic polyimides. The advantages of this procedure include the ease in controlling the loading level of the chromophore and adjusting the polymer backbone rigidity.
Design Strategy for Modification of Chromophores
Chromophores can be designed based on theory and informed by experiment. Arylamine is the basis for this engineering example. The amine is a good donor, there is an acceptor (A) and a π bridge spacer. The letters represent locations where the molecule can be engineered to achieve desired properties:
A = Solubility B = Functionality for non-aggregation C = Donor strength D = Acceptor strength E = Functionality for covalent attachment to polymers F = Stability
In terms of complexity these chemicals are beginning to look less like dyes and more like pharmaceuticals.
Enhanced Stability with Diarylamino Donor
Bob Twieg showed that the decomposition temperature for diazo dyes range from 242 to 322 °C . When they replace the alkyl (aliphatic) group with an aryl (aromatic) group they increase the decomposition temperature 80-100 degrees to 372-406°C. This is because chromophores containing amines with β hydrogens may be particularly susceptible to attack to degradation by singlet oxygen, due to oxidation and subsequent loss of hydrogen. Singlet oxygen is formed when dyes are excited and are able to rip electrons from hydrogen forming radical cations leading to decomposition pathways. Loss of a proton or Hdot is one way these molecules begin decomposition. By eliminating the C-H group you can mitigate against this process.
Aryl amino donors may enhance chromophore photo-oxidative stability.
See Twieg 1994 [1]
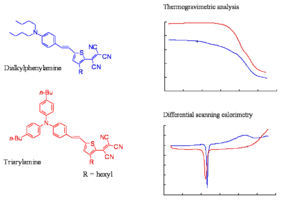
Comparison of Thermal Stabilities
The slide shows the thermogravimetric analysis (TGA) and differential scanning colorimetry (DSC) analysis of a representative triarylamine based chromophore and a dialkylaniline based chromophore. The TGA shows the loss of mass as a function of temperature. The decomposition temperatures of the chromophore of the triarylamine chromophore is at least 50 oC higher than of the dialkylphenylamine chromophore. The triarylamines molecule starts to lose weight about 50 -60 degrees lower than DPA, this means you can work at a higher temperature and use higher Tg polymers. These molecules have one aromatic ring, one less aromatic group, a very strong acceptor, the phenyl groups to make it more stable, and butyl groups on the phenyl groups. These are present to improve the compatibility of the material with the polymer host.
Post Functionalization Approach
Another way to improve the stability of the system is by attaching the material to the host. Polyimid is a high temperature polymer. Alex Jen incorporated functional groups and then added a dye with a hydroxyl group. He attached the dye after the material was polymerized because the conditions for forming the polymer are quite harsh.
High Glass Transition Polymer Approach
Researchers at IBM are making a dye that is extremely stable and then incorporated the dye into the main chain of the polyimide. It can then be poled at 300°C.
It appears that the ultimate achievable stability is related to the inherent flexibility of the polymer backbone and the degree of freedom in the linkage between the chromophore and the polymer (i.e., whether the chromophore is attached in the main chain or in a side chain). Thus, systems with rigid backbones and short rigid linkers will tend to have the highest Tg and also the best temporal stability.
Also, if the rigidity leads to improved temporal stability, it may lead to some degree of crystallinity which can result in significant optical losses due to scattering.
Recently the preparation of a soluble NLO substituted polyimide derivative containing this type of diaryl amino donor head embedded in a polyimide main chain was reported.
The resulting polymer is characterized by an extraordinary stability of the induced polar order, due partially to a high glass temperature (Tg ~ 350°C) of the system. However, it is not surprising that in this polymer, even prior to poling, optical losses due to scattering were relatively large.
Double End Cross-linking Approach
Recently, Dalton et al. have made important advances in poled materials using a "double-ended" crosslinking approach in which the chromophores containing reactive functionalities are at both donor and acceptor ends. Sufficiently different reactive functionalities permit a two-step process to selectively polymerize the precursor polymer first, and then perform the hardening step during the curing/poling process. The OH groups off of the dye and polymer mixed with an isocyanate which reacts with an alcohol to form a bond and make a carbonate. They mix it, cast it into a film, heat it, pole it and allow the isocyanate groups to lock the chromophore into its desired orientation. Dalton has reports long term stability at 90o C for more than 40 days with r33 between 9-17 pm/V
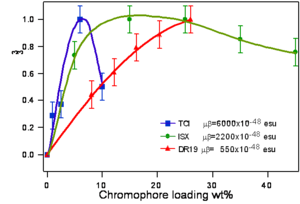
Translating Molecular Optical Nonlinearity into Macroscopic Electro-Optic Activity
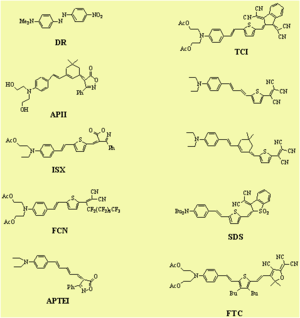
Dalton looked at a series of chromophores and plotted the r- coefficient as a function of the concentration (loading). There was a peak that is due the dipole – dipole interactions were making it difficult to orient the molecules. However with certain molecules were able to stand higher concentrations.
Poling requires a dipole to orient the molecules but large dipoles tend to oppose when they are in close proximity. Thus a balance needs to be found.
See Dalton 1999 [2]. See Marder 1997 [3].
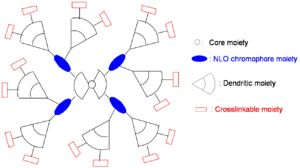
Dendrimer Approach
A dendrimer is alike a tree with many branches. By attaching dendrimeric groups to the chromophore it is possible to have a high concentration without the loss associated with molecules being too close.
Possible variations:
- Enhancement of r33 using more efficient NLO chromophores
- Further minimization of intermolecular electrostatic interaction by more dendrons
- Increase of molecular weight by more branches
- Simplification of synthesis by modular approach
Fluorinated groups decrease optical loss at telecomm wavelength by decreasing the number of C-H bonds which have overtone absorption in this wavelength range. Notice that the molecule at the top has an r33 of 36pm/V at 25% and the other has an r33 at 12 % because the latter molecules are not allowed to get close to each other.
Dendrimer Encapsulated Chromophores
Dendrimer encapsulated chromophores lead to large rlectro-optic voefficient and high thermal stability
Jen and Dalton have created dendrimeric systems with trifluorovinyl groups (shown in red). These can react with each other at high temperature (170 deg) to form cyclobutanes. When the molecules are heated and poled the groups cross link and lock the chromophore into orientation.
This results in a very high E-O coefficient, (r33 = 60 pm/V at 1550 nm) and excellent thermal stability at 85 °C
See Jen 2001 [4]
Performance Comparison of Various NLO Materials
In a guest host system, a simple side chain polymer, and a side chain dendrimer can be grafted together. The R33 goes up and the stability goes up.
See Jen et al. 2002 [5].
Reversibly Thermally Crosslinkable NLO Polymer
These molecules can be poled at a high temperature and then as the temperature comes down a Diels Alder reaction between a diene and a dienophile begin cross linking at a lower temperature. This hardens the lattice. Isolated and low T crosslinking stabilize the alignment. If the polymer is crosslinked you can’t dissolve it and make a film.
A Fully-Functionalized Reversibly Thermally Crosslinkable NLO Polymer
A furan group reacts with a dienophile creating a protected dienophile.. As it heats up the Deils Alder reaction reverses itself creating a dienophile and the side chain of which can react an lock the molecule into place.
Jen's Implementation of Diel-Alder Coupled Poling
Dield-Alder Crosslinked Systems allows you to produce material that is a highly soluble polymer for purification and film casting (because of the protected maleimide, but then can be heated, deprotected, poled and locked into place with cross linking.
These systems have a high R33 are Thermally stable: ~80% of the original value can be retained at 70 °C for more than 250 hrs.
see Jen et al 2002 [6]
Using a monolithic glass poling Alex Jen at UW has Achieved a Milestone: r33 of 500-600 pm/M. Work is in progress to improve thermal stability and mechanical properties of highly efficient EO dendrimers and demonstrate ultra-high EO performance in device stage.
Lessons Learned
These chromophores were not dramatically more non-linear than others, but they optimized the process for orienting them. The take home message is to not get hung up with just one property (eg r33) but to think about all the properties at once. If there is an existing establishing technology a small improvement will not lead people to abandon the old one. A factor of 10 improvement will stimulate change. The STC has lots of people over many institutions who calculate the theory, design the molecules, make the molecules, measure the properties of the molecules, process materials into materials, fabricate them into devices, and then integrate the devices into a system that actually does something. You can be part of a process that is bigger than the specific process you are working on. You will learn to appreciate the challenges that other specialists have and to be able to communicate and work together. This is the reward for working in an interdisciplinary group.
References
- ↑ Twieg, R. J.; et al. Proc. SPIE, 1994, 2143, 2
- ↑ Dalton & Jen et al. J. Mater. Chem., 9, 905 (1999)
- ↑ Marder, Kippelen, Jen, Peyghambarian, Nature, 388, 845 (1997)
- ↑ Jen & Dalton et al., J. Am. Chem Soc. 123, 986 (2001)
- ↑ Jen et al., Adv. Mater., 14(23), 1763 (2002)
- ↑ Jen et al., Adv. Mater., 14, 1763 (2002)
| Previous Topic | Return to Second-order Processes, Materials & Characterization Menu | Next Topic |