Photoelectron Spectrometer XPS and UPS
X-ray Photoelectron Spectroscopy and UV Photoelectron Spectroscopy are techniques for studying the surface characteristic of materials.
What is the Problem?
OLEDs and OPVs consist of thin films of organic materials, sandwiched between contacting electrodes. We need analytical tools which tell us:
- Elemental composition of metal, metal oxide and organic surfaces (top 1-10 nm)
- The molecular state of those elements in that same region
- The frontier orbital energies which control rates of charge transfer, photopotentials, onset voltages, etc.
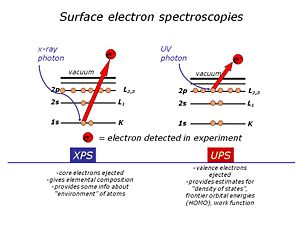
What is our approach? Physics of XPS and UPS
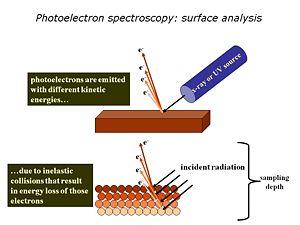
We use high-vacuum surface electron spectroscopies: X-ray photoelectron spectroscopy (XPS)and UV-photoelectron spectroscopy (UPS) to provide the elemental, molecular and energetic information we require about these materials.Surface analysis is carried out in high vacuum spectrometers, with sophisticated sample handling capabilities. The sample is prepared in a chamber to which a variety of devices can be attached. The idea is to keep the surface as clean as possible, and to selectively add monolayers of organic materials to these surfaces, without the need to break vacuum between analyses.The sample is located at the center of the analytical chamber, and positioned so that we can excite it with either X-rays or UV photons.Once the photoelectrons are ejected from the sample, they are collected by a series of focusing lenses, and then separated according to their kinetic energy in a “hemispherical” analyzer. We use either a single “channeltron” detector (UPS) or a multi-channel detector(XPS)
<swf width= "640" height="480">http://depts.washington.edu/cmditr/media/pes.swf</swf>
Data interpretation
Here is a typical “survey spectrum” for a clean gold surface, modified with a single monolayer of a phenyl-terminated alkanethiol. We plot number of emitted photoelectrons on the y-axis, and their binding energy (BE) or kinetic energy (KE) on the x-axis. The photoelectric effect applies (as first described by Einstein) – there is conservation of energy, and you can see that the BE of each photoelectron is related to the excitation source energy, the measured kinetic energy of that photoelectron, and a “work function” of the spectrometer (constant). Each element provides us with at least one photoemission peak, with a distinct BE or KE.