Fluorescent/Phosphorescent Dopants
Return to OLED Menu | Next Topic
Example OLED applications
The Pioneer car stereo was one of the first OLEDs to reach the market with green and blue OLED.
The polymer OLEDs use an emissive layer based on derivatives of polyphenylenevinylene which where spin-cast or deposited with an inkjet printing process. The first of those consisted of two different polmer materials with different ionization potentials and electron affinities to create a heterojunction. These were capped with calcium or magnesium and more recently with aluminum or other more stable metals.
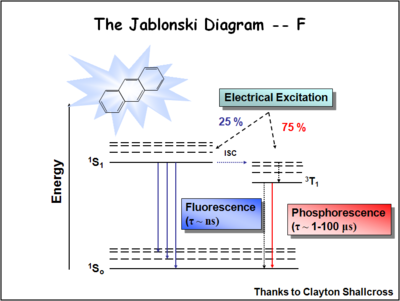
Jablonski Diagram
The Jablonski diagram shows a dopant molecule that can be excited from its singlet state to its first excited singlet state by overlap of its absorption with the emission from the host dye such as aluminum quinolate. The first dopants were primarily fluorescent dopants which shift the energy of the emission of the device slightly more to the red and improve the efficiency and stability of the device. The holy grail is top capture 75% of the energy which exists in the triplet energy. So we need to find dopants that can tap the triplet energy.
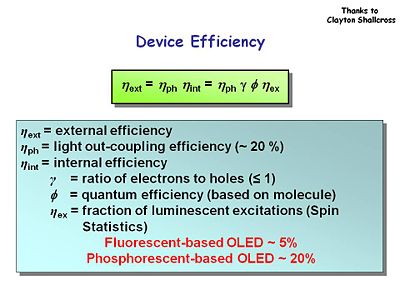
Device Efficiency
External power efficiency is a product of the light out-coupling efficiency and the internal efficiency. With OLEDs only about 20% the light that is created is emitted in the forward direction. The remaining energy is lost in substrate or waveguide modes which go to the side. This has intriguing possibilities for sensors. Internal efficiency is related to the efficiency of all the components in the OLED.
<math> \eta_e _x _t = \eta_ph \eta_int = \eta_ph \gamma \phi_ext</math>
ηext = ηph ηint = ηph γ θ φex
ηext = external efficiency
ηph = light out-coupling efficiency (~ 20 %)
ηint = internal efficiency
γ = ratio of electrons to holes typically ≤ 1. So there is an energy loss due this imbalance.
φ = quantum efficiency of the molecule that is doing the emitting. You want to find molecules with the highest possible quantum efficiency for luminescence.
ηex = fraction of luminescent excitations based on Spin Statistics. Fluorescent OLED have external efficiency of about 5% while phosphorescent OLEDs can go up to 20% efficiency.
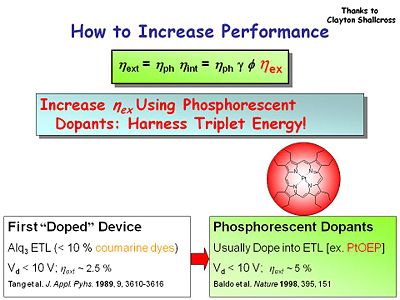
How to Increase Performance
How do you increase performance? First you can balance charge injection. Single anthracene crystal devices had an efficiency of less than 1%. By going to a two layer device the external efficiency jumps to 1%. The first of doped devices use DCM2 a cumarin dye that is used in some dye lasers. It was doped at a high level of 10% works as a energy acceptor from the Alq3 host and it boosted the external efficiency up to 2.5%. It is an orange–red emitter which moves the emission from green to the red part of the spectrum.
Tang et al. Appl. Phys. Lett. 1987, 51, 913-915
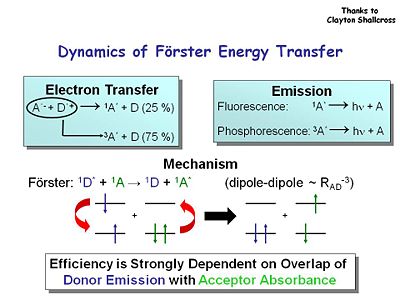
Dynamics of Förster Energy Transfer
You can also increase device performance by increasing φ using energy transfer. Consider the dynamics of Förster energy transfer. The spin statistic dictate that 75% of the electron transfer is in the triplet state. In the case of emission the singlet state gives you fluorescence and phosphorescence comes from the decay of the triplets state back to the ground state. It would be best to capture both types of forms in the devices. Energy efficiency is strongly dependent on the overlap of donor emission with acceptor absorbance.
Because of the weak probability of intersystem crossing from fluorescent to the phosphorescent triplet state these molecules have lifetimes that are rather long, milliseconds and longer. Diplay devices such as OLEDs need to have lifetimes that are shorter.
Phosphorescent dopants such as the heavy metal porphyrin PtOEP pushes the efficiency to 5%. The heavy metal increases the probability of intersystem crossing.
Baldo et al. Nature 1998, 395, 151
The mechanism of triplet energy transfer is Dexter energy transfer involving the exchange of an electron. This has a different distance dependence than Forster transfer, it's much closer in. The right phosphorescent dyes create triplet states at relatively low concentrations of these energy acceptors. The efficiency is strongly dependent on the orbital overlap between the donor and acceptor system. Heavy metal atoms such as platinum and iridium help to mix the singlet and triplet states by spin-orbit coupling.
Tang et al. J. Appl. Phys. 1989, 9, 3610-3616
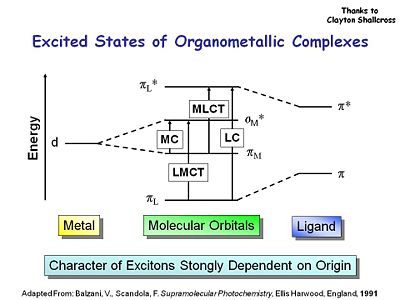
Excited States of Organometallic Complexes
There are a couple of considerations when you work with organometallic complexes. There are ligand to metal transitions in the absorption of these molecules. There are ligand centered transitions, metal to ligand centered and as well as metal centered transitions as well. You have to be careful to understand which of the molecular orbitals you are going to use to maximize the triplet state and the phosphorescence.
See Balzani, V. and Scandola, F. Supramolecular Photochemistry, Ellis Harwood, England 1991, for a review of the rules for design of phosphorescent dopants.
Platinum octoethyl porphyrin (Pt OEP) was the first phosphorescent dopant. The graph shows the host emission from a aluminum quinolate diode. By the time you get to 20% PtOEP there is virtually no emission from the Alq3 and nice red emission from the PtOEP.
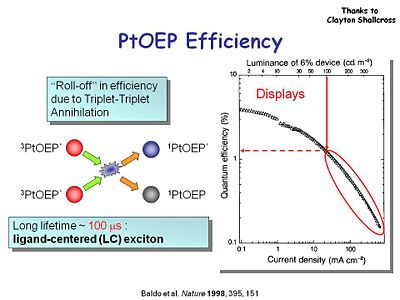
It is important to look at the quantum efficiency for these devices as a function of the applied current density. You want the most light out for least amount of current injected into the system. Pick a threshold luminance of 100 candelas per square meter which is about the about the intensity of a computer screen display. For PtOEP there is a quantum efficiency of 1% at 100 candelas and current density of 10 mA per square centimeter. This is not bad but could be better. Also PtOEP might not be the best choice for an emitter because of its long lifetime of 100 microseconds. We will lose some energy from this phosphorescence because it lasts so long and there are other non-radiative decay processes that suck energy away.