Photochromism
| Previous Topic | Return to Absorption and Emission Menu | Next Topic |
Definition
Photochromism is a reversible transformation of a chemical species induced in one or both directions by absorption of electromagnetic radiation between two forms, A and B, having different absorption spectra. A common example is the sunglasses that turn dark when exposed to sunlight. In order to qualify as photochromic the different forms of the species must have different absorption spectra.
Good References Photochromism by Brown[1]
Photochromism, Molecules, and Systems by Dürr[2]
Photochromism is derived from the Greek words: phos (light) and chroma (color). When light of wavelength lambda1 is exposed to species A it goes to B and when B is exposed to light with wavelength lambda 2 or in the presence of heat, B goes to A.
- <math>A \Leftrightarrow^{\lambda_1}_{\lambda_2, \Delta}B\,\!</math>
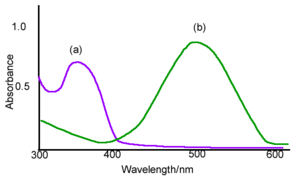
The example spectra of A absorbs around 350 nm and B absorbs around 500nm. One of the ways that you can control the transition is by changing the wavelength of the absorbing light, if they have different spectra. Photochromic materials are used in optical memory in which case you want A to go B and stay there. In this case it desirable that the heat driven transition from B back to A be very small.
The first report of photochromism was given by Fritzsche 1867 [3] who observed the bleaching of an orange-colored solution of tetracene in the daylight and the regeneration of the color in the dark.
Tetracene is a four ring conjugated colored molecule. When the crystals are exposed to sunlight the molecule is broken into the two smaller molecules neither of which absorbs in the visible also known as bleaching. There is transient bleaching, permanent or semi transient types of bleaching.
Interconversion of Isomers
A photochromic conversion of A to B, and B to A can be described by the equations:
- <math>
N_{A\rightarrow B} = N_A .f(\epsilon^A(\lambda), I(\lambda)). \phi_{A \rightarrow B}\,\!</math>
- <math>N_{B\rightarrow A} = N_A .f(\epsilon^B(\lambda), I(\lambda)). \phi_{B \rightarrow A}+ N_B .f’(T)\,\!</math>
Where:
- <math>f(\epsilon^X(\lambda), I(\lambda))\,\!</math> is the fraction of molecules of the species x that is excited by the light per unit time,
- <math>f(\epsilon^X(\lambda), I(\lambda))\,\!</math> is a function of the extinction coefficient of the molecule :<math>[\epsilon^X(\lambda)]\,\!</math> at wavelength l and of the light intensity :<math>[I(\lambda)]\,\!</math> at that wavelength (the absorption spectra)
- <math>\phi_{A\rightarrow B}\,\!</math> is the quantum yield the conversion of A to B
- <math>\phi_{B\rightarrow A}\,\!</math> is the quantum yield of the conversion of B to A
- <math>f'(T)\,\!</math> is the fraction of molecules B that thermally convert to A per unit time, it is a function of temperature [T] (thermal reversal)
The rate of absorption is the rate that molecules go from the ground to the excited state because of the collision with a photon. The rate law for this chemical reaction would be dependent on the concentration of A and the rate of photo flux and the probability of a photo being absorbed. In order for A to go to B it must achieve an excited state. The quantum yield predicts how often the excited state will lead to the formation of B as opposed to decaying, giving off a photo or other non-productive outcomes.
Interconversion – poor coupling
Consider the exited state and ground state potential surfaces. If you assume that the excited state potential well between A and B are not strongly coupled or do not interact then the photon is absorbed into the excited state, over a period of 100s of femptoseconds the molecule relaxes down to the lower vibrational levels within the excited state, and then it can go back down buy fluoresence or non-radiative decay. If the excited state of A goes directly to the ground state of B that will be a temperature independent process because it is extremely fast and because the excited state can slide easily back to the ground state of A because there is no thermal barrier. This pathway is intensity dependent.
References
- ↑ Photochromism,G. H. Brown (Ed.)(Techniques of Chemistry Vol. III),�Wiley-Interscience, New York,1971 (853 pp.).
- ↑ Photochromism, Molecules, and Systems ,H. Dürr and H. Bouas-Laurent (Eds.)(Studies in Organic Chemistry 40), Elsevier, Amsterdam, 1990�(1068 pp.).
- ↑ J. Fritzsche. Comptes Rendus Acad. Sci., Paris, 69,1035 (1867)
| Previous Topic | Return to Absorption and Emission Menu | Next Topic |