Difference between revisions of "Energy vs Charge Transfer at Heterojunctions"
Cmditradmin (talk | contribs) (New page: Return to OPV Menu | Next Topic category:organic solar cell) |
Cmditradmin (talk | contribs) |
||
| Line 2: | Line 2: | ||
[[Current OPV Research Directions|Next Topic]] | [[Current OPV Research Directions|Next Topic]] | ||
[[category:organic solar cell]] | [[category:organic solar cell]] | ||
[[Image:opv_efficiencies.JPG|thumb|300px|]] | |||
These are the types of efficiencies that people have been able to reach. For example, with silicon, fill factors of about 0.8 and conversion efficiency, of 24% can be reached. The Grätzel cell, which is a hybrid cell that has both organic and inorganic components, has a conversion efficiency of about 11%. Michael Grätzel is a professor at the technical school of Lausanne in Switzerland. With polymers, Allen Heeger has recently reported a conversion efficiency of up to 6%. Our goal at this time is to reach the 10% efficiency in organic cells. However, it does not seem to be very easy to achieve. | |||
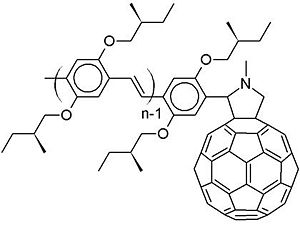
The picture that was given earlier, where the exciton comes to the interface and the electron transfers from the donor to the acceptor, is very simplified. In reality, the process can get much more complicated. That is the reason why the disassociation is not, in most cases, fully understood. One example is oligophenylenevinylene and C60 on a dyad. A dyad simply means that there is an oligophenylenevinylene attached covalently to the c60 derivative. Usually in the literature, people would say you have a pi conjugated oligomer or polymer, and a C60. Since C60 is a very strong electron acceptor, an electron-transfer from the oligophenylenevinylene to the C60 will occur at the interface. | |||
For oligomer/polymer – fullerene heterojunctions photo-induced charge separation is usually assumed to proceed via the formation of a localized excited state on the conjugated oligomer or polymer followed by electron transfer from polymer or oligomer to fullerene. This is consistent with C60 having a very small optical absorption in the visible because of symmetry-forbidden transitions. | |||
[[Image:OPV-C60.jpg|thumb|300px|oligophenylenevinylene – C60 dyads]] | |||
This molecule is the type of molecules that Rene Janssen and his group at Eindhoven University of Technology in the Netherlands have synthesized and factorized. They excited an oligophenylenevinylene segment and there is an exciton within that segment. Instead of having an electron transfer from the excitonic state on the donor to the acceptor, what happened first is an energy transfer to the fullerene. <ref>Janssen et al., J. Phys. Chem B. 104, 10174 (2000); Chem. Phys. Lett. 345, 33 (2001); Appl. Phys. A, 79, 41 (2004). See also: Echegoyen et al., Org. Lett. 3, 335 (2002); Gayathri & Patnaik, Chem. Phys. Lett. 414, 198 (2005)</ref> | |||
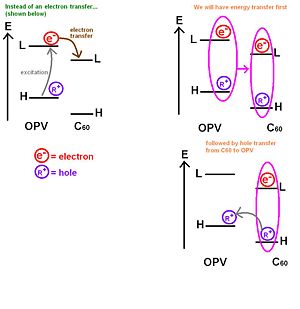
[[Image:Opv-c60_energy_transfer.jpg|thumb|300px|Energy transfers to C<sub>60</sub>]] | |||
If we take a very simple picture with an energy diagram with levels of the C60 and oligophenylenevynylene moieties. Usually, there would be an excitation to the LUMO and the leaving a hole on the HOMO followed by an electron transfer from the OPV to the C60. However, Janssen has shown experimentally that in several dyad systems the entire exciton jumps onto the C60. This is an energy transfer. After the exciton jumps onto the C60 , it becomes favorable for the hole to jump back to the OPV HOMO. In other words, instead of having a direct electron transfer, there is an energy transfer from the OPV to the c60 followed by a hole transfer from the C60 to the OPV. The end picture is the same as the one you would expect if the process only included a simple electron transfer(as previously expected) because the hole would be at the HOMO in the OPV and the electron would be at the LUMO on the C60. However, the rate that describes the energy transfer step plus the hole transfer step could be totally different from the rate corresponding to the direct electron transfer step. | |||
If you were to deal with a direct electron transfer, you want to know how fast and efficient that transfer is compared to the two step process, where you first have an energy transfer followed by a hole transfer. You also want to know how fast and efficient is the two step process with respect to the direct electron transfer process. | |||
The end state is the same, but whether it is reached it more or less efficiently is an open question. So this provides another path for the charge separation, which could be more or less efficient. But the direct path or direct electron transfer might take too long and fail to work. This is an example of how these processes can indeed be more complex. | |||
Energy transfer occurring first, which could happen at long range, can be useful in terms of making the excitons willing to go the interface. In other words, it gives a drift to the excitons instead of a diffusion motion. This is a topic Mike McGehee at Stanford is studying. | |||
This slide just shows that the pole - dipole model that one usually uses to describe energy transfer doesn’t work in this case; you need to go way beyond that simple model. For that system, the calculations indicate that the resonant energy transfer from the OPV segment to the fullerene can occur on a sub-picosecond time scale and thus, it can be extremely fast. | |||
<ref>http://www.stanford.edu/group/mcgehee/publications.html</ref> | |||
== References == | |||
<references/> | |||
Revision as of 11:42, 12 May 2009
Return to OPV Menu | Next Topic
These are the types of efficiencies that people have been able to reach. For example, with silicon, fill factors of about 0.8 and conversion efficiency, of 24% can be reached. The Grätzel cell, which is a hybrid cell that has both organic and inorganic components, has a conversion efficiency of about 11%. Michael Grätzel is a professor at the technical school of Lausanne in Switzerland. With polymers, Allen Heeger has recently reported a conversion efficiency of up to 6%. Our goal at this time is to reach the 10% efficiency in organic cells. However, it does not seem to be very easy to achieve.
The picture that was given earlier, where the exciton comes to the interface and the electron transfers from the donor to the acceptor, is very simplified. In reality, the process can get much more complicated. That is the reason why the disassociation is not, in most cases, fully understood. One example is oligophenylenevinylene and C60 on a dyad. A dyad simply means that there is an oligophenylenevinylene attached covalently to the c60 derivative. Usually in the literature, people would say you have a pi conjugated oligomer or polymer, and a C60. Since C60 is a very strong electron acceptor, an electron-transfer from the oligophenylenevinylene to the C60 will occur at the interface. For oligomer/polymer – fullerene heterojunctions photo-induced charge separation is usually assumed to proceed via the formation of a localized excited state on the conjugated oligomer or polymer followed by electron transfer from polymer or oligomer to fullerene. This is consistent with C60 having a very small optical absorption in the visible because of symmetry-forbidden transitions.
This molecule is the type of molecules that Rene Janssen and his group at Eindhoven University of Technology in the Netherlands have synthesized and factorized. They excited an oligophenylenevinylene segment and there is an exciton within that segment. Instead of having an electron transfer from the excitonic state on the donor to the acceptor, what happened first is an energy transfer to the fullerene. [1]
If we take a very simple picture with an energy diagram with levels of the C60 and oligophenylenevynylene moieties. Usually, there would be an excitation to the LUMO and the leaving a hole on the HOMO followed by an electron transfer from the OPV to the C60. However, Janssen has shown experimentally that in several dyad systems the entire exciton jumps onto the C60. This is an energy transfer. After the exciton jumps onto the C60 , it becomes favorable for the hole to jump back to the OPV HOMO. In other words, instead of having a direct electron transfer, there is an energy transfer from the OPV to the c60 followed by a hole transfer from the C60 to the OPV. The end picture is the same as the one you would expect if the process only included a simple electron transfer(as previously expected) because the hole would be at the HOMO in the OPV and the electron would be at the LUMO on the C60. However, the rate that describes the energy transfer step plus the hole transfer step could be totally different from the rate corresponding to the direct electron transfer step.
If you were to deal with a direct electron transfer, you want to know how fast and efficient that transfer is compared to the two step process, where you first have an energy transfer followed by a hole transfer. You also want to know how fast and efficient is the two step process with respect to the direct electron transfer process.
The end state is the same, but whether it is reached it more or less efficiently is an open question. So this provides another path for the charge separation, which could be more or less efficient. But the direct path or direct electron transfer might take too long and fail to work. This is an example of how these processes can indeed be more complex.
Energy transfer occurring first, which could happen at long range, can be useful in terms of making the excitons willing to go the interface. In other words, it gives a drift to the excitons instead of a diffusion motion. This is a topic Mike McGehee at Stanford is studying.
This slide just shows that the pole - dipole model that one usually uses to describe energy transfer doesn’t work in this case; you need to go way beyond that simple model. For that system, the calculations indicate that the resonant energy transfer from the OPV segment to the fullerene can occur on a sub-picosecond time scale and thus, it can be extremely fast.
References
- ↑ Janssen et al., J. Phys. Chem B. 104, 10174 (2000); Chem. Phys. Lett. 345, 33 (2001); Appl. Phys. A, 79, 41 (2004). See also: Echegoyen et al., Org. Lett. 3, 335 (2002); Gayathri & Patnaik, Chem. Phys. Lett. 414, 198 (2005)
- ↑ http://www.stanford.edu/group/mcgehee/publications.html