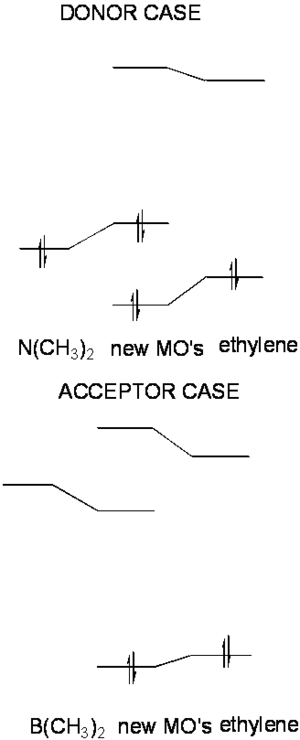Difference between revisions of "Donors and Acceptors"
Cmditradmin (talk | contribs) |
Cmditradmin (talk | contribs) |
||
| Line 1: | Line 1: | ||
[[Main_Page#Molecular Orbitals|Return to Molecular Orbitals Menu]] | [[Main_Page#Molecular Orbitals|Return to Molecular Orbitals Menu]] | ||
[[Image:Donoracceptor.png|thumb|300px|]] | |||
A donor is a high energy orbital with one or more electrons | A donor is a high energy orbital with one or more electrons | ||
An acceptor is a low energy orbital with one or more vacancies: | An acceptor is a low energy orbital with one or more vacancies: | ||
| Line 11: | Line 11: | ||
Because the driving force for an electron in an orbital to be transferred (donated) to another orbital (an acceptor orbital) is related to the difference in energy between the orbital not the absolute energy. | Because the driving force for an electron in an orbital to be transferred (donated) to another orbital (an acceptor orbital) is related to the difference in energy between the orbital not the absolute energy. | ||
In the diagram in each case the electron will end up on the lowest relative energy level. In organic chemistry we often think of CH<sub>3</sub>O and (CH<sub>3</sub>)<sub>2</sub>N as donors even though nitrogen and oxygen are more electronegative than carbon (i.e. their orbital are lower in energy than carbon? | |||
Why is this? | |||
First to be fair we should compare orbitals with equal occupancy and if we compared CH<sub>3</sub>O and (CH<sub>3</sub>)<sub>2</sub>N to (CH<sub>3</sub>)<sub>2</sub>C<sup>–</sup> we would think that the carbanion was a better donor than the methoxy group or the amino group. | |||
[[Image:Donoracceptorcases.png|thumb|300px|]] | |||
Consider what happens to the molecular orbitals of ethylene when we attach donors or acceptor groups to them. | |||
The donor case: | |||
*When the the donor orbital mixes with the molecular orbitals of ethylene it is higher in energy then the the HOMO of ethylene. | |||
*It mixes more with the HOMO than the LUMO because they are closer in energy (perturbation theory). | |||
*As a result of the mixing two new filled orbitals are created and the highest occupied MO is now higher in energy than either the amine orbital or the original ethylene HOMO. This orbital is delocalized and has both carbon and nitrogen character. In fact the orbital will in many ways resemble those of allyl anion. | |||
*The HOMO-LUMO energy is now smaller | |||
The same arguments will hold in reverse for the acceptor case. | |||
Revision as of 15:13, 19 May 2009
Return to Molecular Orbitals Menu
A donor is a high energy orbital with one or more electrons An acceptor is a low energy orbital with one or more vacancies:
- A donor is an atom or group of atoms whose highest filled atomic orbital or molecular orbital is higher in energy than that of a reference orbital
- An acceptor is an atom or group of atoms whose lowest unfilled atomic or molecular orbital is lower in energy than that of a reference orbital.
Why do we use reference orbital?
Because the driving force for an electron in an orbital to be transferred (donated) to another orbital (an acceptor orbital) is related to the difference in energy between the orbital not the absolute energy.
In the diagram in each case the electron will end up on the lowest relative energy level. In organic chemistry we often think of CH3O and (CH3)2N as donors even though nitrogen and oxygen are more electronegative than carbon (i.e. their orbital are lower in energy than carbon?
Why is this?
First to be fair we should compare orbitals with equal occupancy and if we compared CH3O and (CH3)2N to (CH3)2C– we would think that the carbanion was a better donor than the methoxy group or the amino group.
Consider what happens to the molecular orbitals of ethylene when we attach donors or acceptor groups to them. The donor case:
- When the the donor orbital mixes with the molecular orbitals of ethylene it is higher in energy then the the HOMO of ethylene.
- It mixes more with the HOMO than the LUMO because they are closer in energy (perturbation theory).
- As a result of the mixing two new filled orbitals are created and the highest occupied MO is now higher in energy than either the amine orbital or the original ethylene HOMO. This orbital is delocalized and has both carbon and nitrogen character. In fact the orbital will in many ways resemble those of allyl anion.
- The HOMO-LUMO energy is now smaller
The same arguments will hold in reverse for the acceptor case.