Hyper Rayleigh Scattering
Hyper Rayleigh Scattering (aka Harmonic Light Scattering or HRS) is one method for measuring the first hyperpolarizabilityβ. Another method is electric field induced second harmonic generation (EFISH)
Overview
In HRS A dilute sample of a test chromophore is prepared in a solvent. An incident laser generates a second harmonic signal, specifically the frequency double signal from excitation of the sample molecules. This can be related to the beta of the sample using this formula:
- <math>\I^ {2 \omega}_{HRS} = [ (I^\omega_0 \cdot T^\omega_{solv})^2 ] (N_{solv} \langle \beta^2 _{solv} \rangle + N_{sam} \langle \beta^2_{sam}\rangle} )\,\!</math>
See Firestone 2004 [1].
There are a number of ways the HRS characterization method is used in non-linear optics research.
The dielectric properties of the solvent influence the β (solvatochromatism). One area of research involves predicting and solvent effects so as to better understand the performance of molecules when densely packed in materials. Attempts have been made to calibrate HRS measurements with EFISH measurements of beta though they are based on different tensor elements.
See Wikipedia on Rayleigh Scattering
See also Density Functional Theory
See Wikipedia on Raman Scattering
Technique
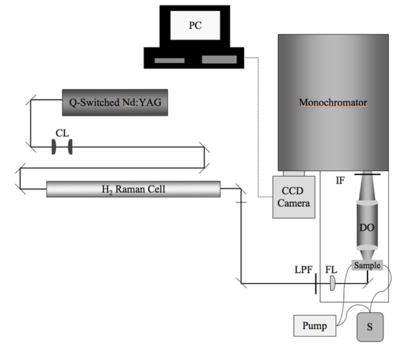
The HRS measurement of β is a specialized device custom-built on an optics table. The components and configuration can change significant from site to site. It is important to understand the significance of each portion of the setup. One possible HRS experimental setup is described here. (Press > to Play the video)
<swf width="550" height="430">http://depts.washington.edu/cmditr/media/rayleigh.swf</swf>
At this point a number of mathematical manipulations and comparisons can be done, for example comparing the sample HRS to a known reference sample with known beta, or internal referencing of the solvent contribution of beta.
Significance
References
- ↑ K. A. Firestone, P. Reid, R. Lawson, S. H. Jang, and L. R. Dalton, “Advances in Organic Electro-Optic Materials and Processing,” Inorg. Chem. Acta, 357, 3957-66 (2004)