The First OLEDs
| Previous Topic | Return to OLED Menu | Next Topic |
Return to OLED Menu | Next Topic
The first OLEDs were green as seen in this early example from the University of Arizona. The image on the right is so bright that is appears white.
The First (“Heroic”) Experiments to Generate Light From Organic Materials:Top and Bottom Electrodes/Rectifying Junctions
The first experiments to generate light from organic materials can be traced back to the late 1950s and early 1960’s at places like RCA. These studies were driven by the hypothesis that these materials would act as efficient light sources and would also serve as organic photoconductors.
See Helfrich [1]
People would take highly purified anthracene and cut it very fine, approximately 5mm thick. They would connect these to electrodes and apply between 100 and 1000 volts. If the system did not short there would be light emission. Often there would be a lightning bolt like discharge and you produce graphite instead of light. The high voltages were needed to overcome the internal resistance to charge migration in the organic solid (RS). The relatively large thickness of these slices was an experimental necessity – pin-hole-free ultrathin film deposition technology was not yet available for organic materials. Emission was seen occurring near the cathode, suggesting that charge recombination occurred closer to this electrode than the center of the “device”.
<embed_document width="55%" height="400">http://depts.washington.edu/cmditr/media/OLED4_firstexperiments.pdf</embed_document>
Next they brought the thickness down to 50 microns and used a semitransparent gold electrode. How is light generated? At the anode there is the removal of an electron to create a cation radical state. At the cathode is the addition of an electron to create the anion radical state. These charges need to hop through the organic crystal in order to meet each other somewhere near the center of the device. They undergo an electron transfer between this reduced donor and the oxidized receptor. There is enough excess free energy generated to create the emissive state of anthracene. The key piece is that in the early devices the rate of transport of the positive cation radical or hole state and the transport of the electron rich state where not equal so the recombination occurred near one of the contacting electrodes. This proves to be a real problem for the efficiency of the device.
The next step in the evolution of the OLED was to use much thinner slices of anthracene, with a semi-transparent gold anode and a silver paste cathode. Lower drive voltages were required, and light emission through the anode was observed. Despite the relatively high external quantum efficiency of these devices, it was clear that substantial materials development would be necessary before real display devices would be realized. The cited review by Dresner shows a substantial understanding of the physics of these light emitting organic thin films, which extrapolates directly to our understanding of OLEDs today.
The First “Practical” Organic Light Emitting Diodes
The first practical OLEDs was built in the late 1980’s by Ching Tang and Andy Van Slyke at Kodak. This was a revolution for the technology.
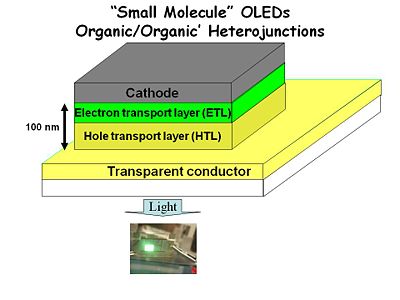
C.W. Tang and coworkers at Kodak saw an opportunity to create the first organic light emitting diodes by using spin casting and sublimation deposition of the organic components of the device, with patterned electrodes top and bottom. Each organic layer was ca. 50 nm thick, the Mg:Ag top cathode was of comparable thickness. Transparent conducting oxide (TCO) thin films on glass served as the anode and provided for light to be emitted in a useable fashion for displays. The keys to successful development of these first devices was control of organic film deposition to create a pin-hole free film (high RP) which was thin enough to yield a lower series resistance than the first anthracene devices, providing for light emission with drive voltages under 50 volts.
They started with a glass or plastic substrate with a transparent conductor such as indium tin oxide which can be highly doped and be highly conductive yet maintain transparency in the visible wavelength region. They deposited a hole transport layer typically some tri arylamine or in the early versions a polymer called poly vinyl carbazol; something that is easily oxidized at this transparent conductor.
Next we add an electron transport layer and the total thickness has been reduced down to 100 nm. This was a technological advance because you were no longer having to slice single crystal anthracene really thin with a razor knife instead you are vapor depositing molecules in a vacuum system on top of this transparent conductor. This also solved another problem. When you deposit two successive layers there are fewer pin holes that create a dead short circuit between the anode and cathode.
Finally the top electrode is something like aluminum, magnesium, silver alloy or even an electropositive metal such as calcium. The hole transparent layer was a spin coated polymer. This produced about 15 candelas per square meter. A typical CRT computer screen operates with 100-200 candelas per square meter. So this was a dim but visible light. Aluminum tris quinalate was used as both the electron transport and emissive layer.
- Vacuum deposition enabled thin electron transport layer
- Hole transport layer was spin-coated polymer: 10 – 20 V, 15cd/m2 brightness
- All vacuum device: 10 – 20 V, 100 cd/m2 using Alq3 emission layer