Work Function of Metals
Work Function - Physical Description
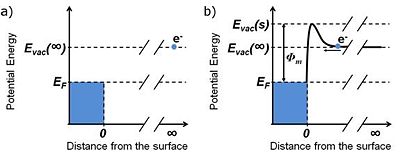
Let us consider an electron at rest in a vacuum at an infinite distance from a metal surface, as depicted in Figure 1a. The total energy, Etot, of such an electron is equal to its potential energy, which can be defined after Cahen and Kahn as the vacuum level at infinity, Evac(∞).[1]. Let us supply the electron with some kinetic energy, Ek, and allow it to travel towards the surface. The total energy of the electron outside the metal can be expressed as the sum of its potential energy, EV, and kinetic energy, Ek:
- <math>E_{tot} = E_V + E_k\,\!</math> Equation 1
As the distance between the electron and the metal surface decreases the potential energy of the electron will increase, thus slowing it down, according to Equation 1 (see Figure 1b). If the kinetic energy is sufficiently large to overcome the potential barrier at the metal / vacuum interface the electron will go over the barrier and then will rapidly lose its potential energy due to the interaction with the positively charged ion lattice within the metal, resulting in the increase of kinetic energy. Once the electron is within the metal Equation 1 no longer holds as the interactions with other electrons and the ion lattice result in energy dissipation and thermal equilibration. The electron is now trapped within the metal.
A similar thought experiment can be performed in the opposite direction. Let us choose an electron from one of the highest occupied energy levels at the particular temperature of the system. The potential energy of this electron is approximately at the Fermi level, EF. In terms of Fermi – Dirac statistics the Fermi level is defined as the energy at which the probability of finding an electron is 0.5 for a system at thermal equilibrium. For metals at room temperature the probability of finding an electron below the Fermi level is close to unity.I[2] [3]
The electron is then supplied with some kinetic energy, E'k, sufficiently large to overcome the potential barrier existing at the metal surface. Right after escaping the metal, the greatly slowed-down electron has a potential energy which can be defined as the vacuum level close to the surface, Evac(s).1 The drop in the kinetic energy of the electron caused by the potential energy barrier is defined as the work function of the metal surface, φm: 1,2
- <math>\Phi_m = E_{vac}(s) - E_F\,\!</math> Equation 2
After escaping from the metal the electron is moving away further from the surface experiencing acceleration as the potential drops to the value of Evac(∞).
It is important to describe the physical origin of the potential landscape illustrated in the above thought experiment. As already mentioned, the sharp potential drop the electron experiences when it enters the metal is related to the electrostatic attraction force between the negative charge of the electron and the positively charged ion lattice of the metal. However, at the vacuum / metal interface the electron experiences a potential energy barrier. This implies that in that region of space there must be a repulsive force that acts upon the electron. Indeed, the lack of positively charged metal ions on the vacuum side of the aforementioned interface causes a negative charge to exist just outside of the metal and, conversely, an uncompensated positive charge within the metal. The spilling of the electronic density outside of the solid causes a formation of a sheet of dipoles, which are often referred to as the surface dipoles.1-3
Work Function of Metallic Surfaces – Methods of Measurement
The work function of solid surfaces can be determined experimentally using absolute or relative approaches. Absolute methods allow one to measure the work function value directly. Here, the electrons in the metal are supplied with sufficient kinetic energy to overcome the barrier at the metal / vacuum interface, and can thus escape the metal, and the work function can be obtained from the resulting electric current. Absolute methods include measurements based on thermionic emission, field emission, and the photoelectric effect. Briefly, in the thermionic emission method electrons are ejected from the material after receiving sufficient thermal energy to overcome the energy barrier at the metal / vacuum interface. The appropriate thermal energy is supplied by incremental heating of the sample to temperatures at which the Fermi-Dirac distribution of the electrons in the metal allows for substantial population of electrons at energies higher than the interface energy barrier. The resulting electric current is measured as a function of temperature; this allows one to extract the work function of the surface. The temperature range used in the thermionic emission method is often very high (thousands of Kelvins), making this method of limited value for studying materials and surfaces which are unstable at high temperatures.4 The field-emission method utilizes an electric field to accelerate the electrons inside of the metal to kinetic energies sufficiently high to overcome the interface barrier. The resulting electric current is analyzed as a function of the applied field and the work function is calculated.[4][5]
Photoelectric-effect-based methods use light, typically in the UV range, as the source of energy for the electrons. As in other absolute methods, the resulting electric current (here called photocurrent) is analyzed. Figure 2 shows the energetics of a photoelectric-effect-based measurement of the work function. In this experiment the electrons are provided with a known energy, hν (red arrows in Figure 2). Electrons with sufficient kinetic energy to overcome the barrier at the interface are able to escape the metal – these are represented with the blue box in Figure 2. These photoelectrons then travel away from the metal surface experiencing the potential depicted with the thick black line in Figure 2. As the electrons move further away from the surface their kinetic energy increases, according to Equation 1. The generated photocurrent is then measured as a function of the photoelectron kinetic energy. Two important features are present in a typical plot of photocurrent – kinetic energy. First, a sharp onset at low photoelectron kinetic energy, Emin is present. As already mentioned, this onset defines the lowest energy electrons able to overcome the work function of the surface.
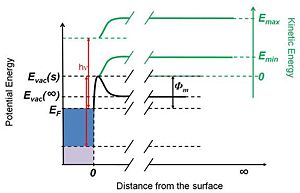
The second feature is the high kinetic energy onset of the photocurrent, Emax, and it is a manifestation of the electron population around the Fermi level of the metal; i.e., since there is an abrupt decrease in the electron population above the Fermi level, there are essentially no photoelectrons with Etot > EF + hν right after ejection from the surface. Using the definition of the work function in Equation 2 (see also Figure 1b), it follows that φm = Emin + hν - Emax. In practice the photoelectrons are additionally accelerated by an external electric field, which has to be taken account in the calculations. Thus, the work function can be obtained from the onsets of the photocurrent as a function of the kinetic energy of the photoelectrons.1,2
Relative methods employ a reference made of a material with a known work function and focus on measuring differences in electrical quantities between the studied material and the reference. These methods include diode methods and condenser methods (Kelvin probe) and will not be discussed further.5
Table 1 shows experimentally measured work-function values for a variety of metallic surfaces. The presented data show a variation of the work function in the range of 2.7 – 5.65 eV, revealing that the chemical composition of the surface has a large effect on the work function value.
Work Function Values
Table 1. Experimentally measured values of work function for different metals.*
#
|
* From photoelectric-effect-based measurements. Values taken from ref. 6.
References
- ↑ Cahen, D.; Kahn, A. Adv. Mater. 2003, 15, 271-277
- ↑ shii, H.; Sugiyama, K.; Ito, E.; Seki, K. Adv. Mater. 1999, 11, 605-625.
- ↑ Ishii, H.; Sugiyama, K.; Ito, E.; Seki, K. Adv. Mater. 1999, 11, 605-625.
- ↑ Murphy, E. L.; Good, R. H. Phys. Rev. 1956, 102, 1464
- ↑ Hölzl, J.; Schulte, F. K.; Wagner, H. Solid surface physics; Springer-Verlag: Berlin, 1979; Vol. 85
- ↑ Nieuwenhuys, B. E.; Van Aardenne, O. G.; Sachtler, W. M. H. Chem. Phys. 1974, 5, 418-28.