Electronegativity and Bonding Between Atoms
Return to Molecular Orbitals Menu | Next Topic
Electronegativity describes how much an atom hold its electrons tightly. An atom that is less electronegative will be more likely to give up its electrons. The electronegativity of atoms determines the degree that electrons are transferred between molecules or shared between bonding atoms.
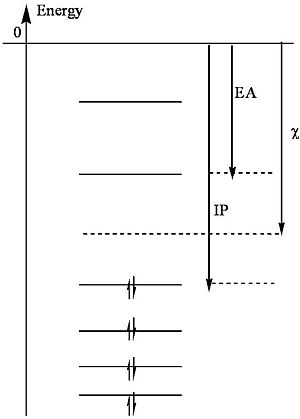
Mulliken presents a useful definition of electronegativity as the average of the ionization potential (IP) and the electron affinity (EA).
<math>\chi = \frac{IP + EA}{ 2}\,\!</math>
Note that the ionization potential is the energy required to remove an electron from an atom (to vacuum) and the electron affinity is the energy released when an atom captures an electron (into the lowest unfilled orbital).
The more electronegative the atom the lower the energy of the orbital within a given row.
Here is a summary of the different kinds of bonds.
| header Ionic Bonding | header Polar Covalent Bonding | header Covalent Bonding |
|---|---|---|
| Forms salts that dissociated in water | Forms bonds which have dipole | Forms bonds with no dipole |
| Between atoms of the far left and far right of the periodic table | Betwen atoms of different columns usually on the right side of the table | Between two like atoms |
| Large differences in electronegativities | Moderate differences in electronegativities | No difference in electronegativities |
| Very unequal sharing, electron is transferred form one atom to another | Unequal sharing of electrons | Equal sharing of electrons |
| Na+Cl- | C-F bond | F-F bond
|