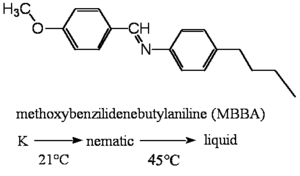Difference between revisions of "Liquid Crystals"
Cmditradmin (talk | contribs) m (→References) |
Cmditradmin (talk | contribs) m |
||
| Line 5: | Line 5: | ||
</tr> | </tr> | ||
</table> | </table> | ||
[[Image:Solar_cell.png|thumb|300px| ]] | |||
Liquid crystalline materials have optical properties that are intermediate between perfectly ordered crystals and completely unordered liquids. The molecules involved are able to refract light differently depending on their orientation. This can be changed by magnetism, by an electrical field, or by changing temperature. In this introduction to liquid cyrstals we will apply ideas including index of refraction, polarizability, crystal lattices and intermolecular forces. | Liquid crystalline materials have optical properties that are intermediate between perfectly ordered crystals and completely unordered liquids. The molecules involved are able to refract light differently depending on their orientation. This can be changed by magnetism, by an electrical field, or by changing temperature. In this introduction to liquid cyrstals we will apply ideas including index of refraction, polarizability, crystal lattices and intermolecular forces. | ||
Revision as of 14:49, 14 June 2012
| Return to Liquid Crystal Menu | Next Topic |
Liquid crystalline materials have optical properties that are intermediate between perfectly ordered crystals and completely unordered liquids. The molecules involved are able to refract light differently depending on their orientation. This can be changed by magnetism, by an electrical field, or by changing temperature. In this introduction to liquid cyrstals we will apply ideas including index of refraction, polarizability, crystal lattices and intermolecular forces.
Liquid Crystal Applications
Liquid crystals are widely used for electronic displays and TVs. In these devices an array electrically controlled LC pixels are placed in front of colored light sources or reflect light differentially as in a calculator. An LC filter can also be used to modulate a projected light source as in an LCD projector. Liquid crystal paint can be used to indicated temperature and pressure which can then be analyzed optically.
See also Wikipedia on liquid crystals
See also Western LC website
See also Case Western Virtual Lab
What is a liquid crystal?
Liquid crystals are anisotropic materials. This is important for their function in LC displays.
In a crystal there is periodicity of a lattice in all three directions. In a liquid there is neither orientational or positional order.
A liquid crystal phase or mesomorphic phase is a phase comprising molecules having a higher degree of orientational order than is found in a liquid and less positional order than is found in a crystal. Under certain conditions they can flow.
There are many classes of liquid crystals having differing degrees of order. We need to ask if a molecule is capable of being liquid cyrstalline under certain conditions. Some molecules can be crystalline, liquid crystalline (and exist in different phases of liquid crystalline) and isotropic, depending on the conditions.
As shown in the diagram to the right there are discrete phase transitions between the crystal phase, the LC phase and the liquid phase. At each phase transition there is a corresponding heat of enthalpy for the transition. Much of the research in this area is german, as a result the letter K is often used to denote the cyrstalline phase (krystal in german). Nematic refers to the liquid cyrstalline phase (abbreviated N). Liquid phase may be abbreviated as l or I (for isotropic). Under the arrrow is temperature at which there is a phase transition. These transitions are usually quite sharp.
History of Liquid Crystals
One of the first papers about liquid cyrstals appeared more than 100 years ago.
1888 - F Reinitzer ([1]) observed two melting points in cholesteryl benzoate. Today these are known as cholesteric liquid crystals.
1911 - C. Mauguin ([2]) performed and described the first electro-optical experiments involving a twisted nematic phase.
1922 - M. G. Friedel ([3]) recognized three kinds of LC phases and introduced a new terminology.
- Smectic "soapy"
- Nematic "thread"
- Cholesteric (twisted nematic)
Shampoo in a bottle or soap in dish may appear irridescent. This is called a lyotropic liquid crystal in which phase transition is a function of concentration. Thermotropic liquid crystals have a phase change that is dependent on temperature. Lipid bilayers are phase transitions that are lyotropic.
1927 - W. Friederickz et al. ([4]) described the influence of electrical and magnetic fields on smectic, nematic and cholesteric LCs. Marconi Wireless Telegraph Co was awarded a patent on a light valve in 1936.
1959 - W. Maier and S. Saupe ([5]) published what is now known as the Maier-Saupe theory of liquid crystals which suggests the temperature at which material move from liquid crystalline to isotropic. It is summarized in the equation:
- <math>T_{is} = A/4.55kv^2\,\!</math>
Where: A is a molecular parameter including the polarizability anisotropy, k is Boltzmann's constant, v is the molar volume.
1963 - W. Richards and G. Heilmeier (RCA Corporation)([6]) foresaw "TV on a wall".
Then there was a rediscovery of electro-optical effects and their applications in LC display:
1969 - Dichroic dye; host guest ([7]).
1969 - Phase change displays ([8]).
1973 - Twisted nematic field effect ([9]).
1973 - G.W. Gray et al. ([10]) developed LCD technology based upon cyanobiphenyls.
1977 - S. Chandrasekhar et al. ([11]) discovered discotic liquid crystals.
1991 - Pierre-Gilles de Gennes, Nobel Prize Physics 1991, studied how extremely complex forms of matter behave during the transition from order to disorder. He showed how electrically or mechanically induced phase changes transform liquid crystals from a transparent to an opaque state, the phenomenon exploited in liquid-crystal.
So there is a long research history in liquid crystals and much current work as well.
References
- ↑ Montash. Chem. 1888, 9, 421
- ↑ Bull. Soc. Fr. Min. 1911, 34, 71
- ↑ Ann. Phys. 1922, 18, 273
- ↑ Z. Phys. 1927, 42, 532
- ↑ Z. Naturforsch. 1959, 13a, 564
- ↑ J. Chem. Phys. 1963, 39, 384
- ↑ G. H. Heilmeier et al. Appl. Phys. Lett. 1969, 13, 46
- ↑ G. H. Heilmeier et al. Proc. IEEE. 1969, 57, 34
- ↑ J. L. Fergason US patent # 3,731,986, 1973
- ↑ Electron. Lett. 1973, 9, 130
- ↑ Pramana 1977, 9, 471
| Return to Liquid Crystal Menu | Next Topic |