Difference between revisions of "Electromagnetic Radiation"
| Line 1: | Line 1: | ||
[[Main_Page#Luminescence and Color|Return to Luminescence Menu]] | | [[Main_Page#Luminescence and Color|Return to Luminescence Menu]] | | ||
[[Electromagnetic Spectrum|Next Topic]] | [[Electromagnetic Spectrum|Next Topic]] | ||
[[Image:Emwavepropagation.jpg|thumb| | [[Image:Emwavepropagation.jpg|thumb|400px|]] | ||
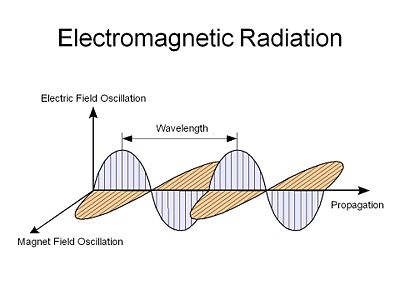
==Electric an Magnetic waves== | ==Electric an Magnetic waves== | ||
Light is an electromagnetic radiation, an electric field that oscillates in both time and space along with a corresponding orthogonal magnetic field that oscillates with the same spatial and temporal periodicity. This was first described by Maxwell. | Light is an electromagnetic radiation, an electric field that oscillates in both time and space along with a corresponding orthogonal magnetic field that oscillates with the same spatial and temporal periodicity. This was first described by Maxwell. | ||
Revision as of 15:37, 4 May 2009
Return to Luminescence Menu | Next Topic
Electric an Magnetic waves
Light is an electromagnetic radiation, an electric field that oscillates in both time and space along with a corresponding orthogonal magnetic field that oscillates with the same spatial and temporal periodicity. This was first described by Maxwell.
When light gets into a material it interacts with the charged particles within the atom. Although both magnetic and electric field can be absorbed by materials, the interaction of the field of light is usually about 105 stronger than that of the magnetic field and thus to the first approximation we can concern ourselves only with the interaction of the electric field with matter. When the electric field of light gets into a material and it cause the electrons to move.
Formally, the term “light” is strictly applicable to the visible portion of the electromagnetic spectrum, however we will use a looser definition that includes the entire visible and non-visible spectrum.
Light behaves both as wave with properties of wavelength and frequency and as particle in that it is quantized in discrete packets known as photons.
Wavelength (λ) is measured in nanometers (nm)
Period T in seconds
Frequency (ν) is 1/T measured in Hertz
<math>c=\lambda \nu\,\!</math>
Light waves
The light waves can be described as: X is direction of travel <math>F_o\,\!</math> is the amplitude
<math>F = F_o sin [(2\pi/ \lambda) (x-vt) + \phi]\,\!</math>
<math>=F_o sin [k(x-\lambda vt) + \phi]\,\!</math>
<math>=F_o sin [kx- \omega t + \phi]\,\!</math>
<math>K \equiv \frac{2\pi} {\lambda}\,\!</math> = wave vector or wavenumber in cm-1
<math>\omega = 2\pi \nu\,\!</math> = angular frequency ( in radians/s)
<math>\phi\,\!</math> is the phase constant that accounts for a non-zero amplitude at the origin of the plot.
The particle nature of light
The energy of the light beam is concentrated in the particles called photons.
<math>E = \frac{h}{\nu}\,\!</math>
h is plancks constant = 6.6 x 10-34 J·S in SI units
Which can be divided by the charge of the electron 1.6 x10-19 coulomb to give a microscopic equivalent of
E= 4.14 x 10-15 eV·s
The speed of light in a vacuum at approximately 3 x 108 m/s