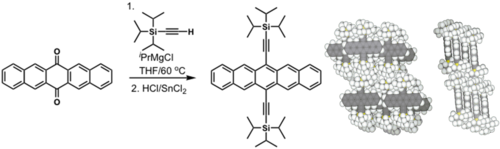Difference between revisions of "Synthesis of Organic Semiconductors"
Cmditradmin (talk | contribs) |
Cmditradmin (talk | contribs) |
||
| Line 182: | Line 182: | ||
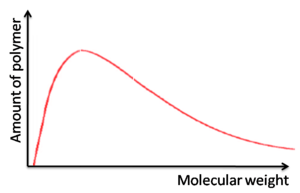
=== Molecular weights of polymers === | === Molecular weights of polymers === | ||
[[Image:Polymer_mw.png|thumb|300px|]] | |||
=== Small molecule vs. Polymer semiconductors === | === Small molecule vs. Polymer semiconductors === | ||
Revision as of 15:30, 12 February 2010
Design criteria
- HOMO/LUMO levels and bandgap
-Controlled by type of conjugated system, electron donating/electron withdrawing groups
- Solid state packing/self-assembly
-Presence and position of substituents
- Solubility
-Introduction of substituents
- Volatility
- Ease of synthesis
HOMO/LUMO level control
- The HOMO increases in energy with increasing conjugation length.
- The LUMO decreases in energy with increasing conjugation length.
- The band gap (Eg) is decreases with increasing conjugation length.
- Polymer is more susceptible to electrophiles because of its higher HOMO. ie. more reactive.
Effect of electron donating and electron withdrawing substituents
Electron donating groups increase the energy levels.
Electron withdrawing groups decrease the energy levels.
Effect of polymer structure
Twists in the structure generally decrease the effective conjugation length and therefore increase the bandgap.
Substituents
Bulky substituents will increase solubility making the material easier to process.
However, in the solid state, bulky substituents will disrupt the packing of molecules/polymers therefore decreasing charge mobility through materials.
The substituent often has to be altered through trial and error to obtain material with the appropriate HOMO/LUMO levels, solubility, and optoelectronic performance.
P-type small molecule/oligomer synthesis
Examples of p-type molecules: Pentacene
Excellent TFT performance Best TFTs give > 5 cm2/(V s), ION/IOFF = 106
Insoluble: Devices fabricated by vacuum sublimation
Pentacene is oxygen and light sensitive
Efforts to solubilize pentacene: Silyl modified pentacene
Solution processed TFTs: > 5 cm2/(V s)
see Anthony 2001[1]
see Park 2006 [2]
Soluble precursor approach
Combines best of both worlds by providing material that is soluble, but has good packing once solubilizing group is removed.
OTFTs
μ = 0.1 cm2 / V⋄s
ION / IOFF = 2 x 105
see Weidkamp 2004 [3]
see Afzali 2002 [4]
Examples of p-type molecules: Oligothiophenes
Packing aided by liquid crystalline-like behavior of alkyl chains Sparingly soluble in hot organic solvents
see Lovinger 1998[5]
Soluble precursor route
- Precursor is highly soluble in organic solvents
- Heating burns off the solubilizing groups, anneals thiophenes into terraced structures
OTFTs: μ= 0.05 cm2 / V⋄s; ION / IOFF = 105 after thermal treatment
see Murphy 2004 [6]
N-type small molecule/oligomer synthesis
N-type materials
Most organic materials are p-type.
Two procedures are generally used to make a material n-type.
-Decrease LUMO level of material by introducing electron withdrawing groups eg. naphthalene derivatives
-Decrease LUMO level by introducing strain eg. C60 derivatives
Examples of n-type molecules
Aromatic bis-imides
One of the early organic n-FET successes.
see Katz 2000 [7]
see Würthner 2004 [8]
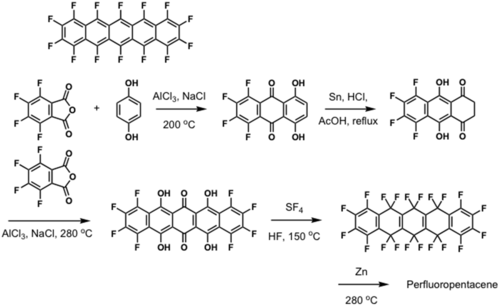
Fluorinated pentacene
μ = 0.22 cm2/Vs and Ion/Ioff =105
see Y. Sakamoto; T. Suzukil; M. Kobayashi; Y. Gao; Y. Fukai; Y. Inoue; F. Sato; S. Tokito J. Am. Chem. Soc., 2004, 126, 8138–8140.
C60 derivatives
Phenyl C60 Butyric Acid Methyl Ester
(PCBM)
Thienyl CBM (ThCBM)
See Lacramioara 2006 [9]
Single precursor p & n-type material
N-type OTFT μ = 0.08 cm2/Vs and Ion/Ioff =106
P-type OTFT μ = 2 × 10-4 cm2/Vs and Ion/Ioff =104
see Yoon 2007 [10]
Review of polymers
Step growth vs. Chain growth polymerizations
Step growth
Broad molecular weights
- Molecular weight is heavily dependent on the purity of the monomer
- Leads to batch-to-batch variability
- Optoelectronic properties vary which means fluctuating electronic device performance
Chain growth
- One monomer at a time adds to the growing polymer chain.
- Under certain conditions, the polymerization can be controlled to produce specific molecular weights with narrow polydispersities (living polymerization)
Molecular weights of polymers
Small molecule vs. Polymer semiconductors
- Small molecules have well-defined molecular weights which lends itself better to provide crystalline packing.
- Polymers generally contain amorphous domains which reduces charge transport.
- Polymers are more amenable to room temperature solution processing. Although both small molecules and polymers can be solubilized, polymers tend to make smoother, more continuous films.
Semiconducting polymers
Semiconductivity in polymers can be achieved in two ways:
- By having pendant small molecule semiconductors attached onto an insulating polymer backbone.
- By having a conjugated polymer.
- Polymers with pendant groups tend to show poorer charge mobility because it is difficult to organize the polymer such that the pendant groups stack well.
- But, easier to perform a controlled polymer synthesis on polymers with pendant groups using, for example, ATRP, ROMP, and NMRP.
Common conjugated polymers
P-type polymer synthesis
Polythiophenes
For comprehensive review on polythiophenes: R. D. McCullough, Adv. Mater. 1998, 10, 93
Historical progression of polythiophenes
Initially, conjugated polymers were synthesized by oxidative coupling reactions.
But oxidative coupling can lead to defects. Eg. instead of the required 2,2 coupling, 2,3 coupling can also take place.
Dehalogenation routes were also attempted.
Better than oxidative coupling because 2,3 coupling can be avoided.
However, both routes still suffer when solubilizing groups are added.
Regiorandom polymers end up being synthesized when a regioregular HT-HT polymer is desirable.
By differentiating the two ends of the substituted thiophene, which can be done cleanly, it is possible to do a cross coupling reaction and thereby synthesize truly regioregular polyalkylthiophenes.
McCullough method: J. Chem. Soc. Chem. Commun. 1992, 70-72 Rieke method: J. Am. Chem. Soc. 1992, 114, 10087
Improving on regioregular poly(3-hexylthiophene) (P3HT)
Poor TFT performance when devices are fabricated in air. (IOFF high due to O2 doping)
B. S. Ong; Y. Wu.; P. Liu; S. Gardner, J. Am. Chem. Soc., 2004, 126, 3378.
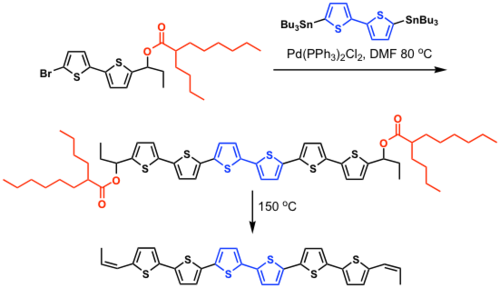
Fused ring polythiophenes
M. Heeney; C. Bailey; K. Genevicius; M. Shkunov; D. Sparrowe; S. Tierney; I. McCulloch J. Am. Chem. Soc., 2005, 127, 1078-1079.
Synthesized via soluble precursor route
Wessling, J. Polym. Sci. Polym. Symp. 1985, 72, 55
Wessling, 1968 (?!), US Patent 3,401,152 and 1972, US Patent 3,706,677
Soluble PPV
3-(bromomethyl)heptane, KOH, C2H5OH, reflux formaldehyde, conc. HCl, dioxane KOC(CH3)3, THF
Wudl et al. ACS Symp. Ser, 1991, 455; US Patent 1990, 5,189,136
More p-type polymers: Polyfluorenes
Polyfluorene: Originally synthesized in 1989 Fukuda et al. Jpn. J. Appl. Phys. 28, L1433, 1989
Adv. Funct. Mater. 15, 981, 2005
More p-type polymers: Polyphenylenevinylenes (PPV)
Polyfluorenes: obtained blue polymer for LEDs
Originally, the emission at approx. 550 nm was thought to be a results of aggregation. Bulky substituents were added to polyfluorene to reduce green emission and create “blue” polymer.
J. Am. Chem. Soc., 123, 6965, 2001
List and Scherf et al. Adv. Mater. 14, 374, 2002
But it was realized that the red-shifted emission was due to keto defects within the polymer.
Polymer design was altered so that there would be a silicon bridge rather than a carbon bridge to prevent keto defects from forming.
Chan and Holmes et al. J. Am. Chem. Soc. 127, 7662, 2005.
N-type polymer synthesis
N-type polymers
Rare but growing area of research. Highly ordered Lamellar packing μ = 0.10−0.16 cm2/(V s), Ion/Ioff = 107 Devices stable in air for >5 months
H. Usta; A. Facchetti; T. J. Marks
J. Am. Chem. Soc., 2008, 130, 8580.
=== A. Babel and S. A. Jenekhe J. Am. Chem. Soc., 2003, 125, 13656.
Napthalene based polymers ===
JACS, 2009, 8-9.
Ambipolar polymers
F. S. Kim and S. A. Jenekhe et al. Adv. Mater., Vol. 21, P. 1-5, 2009
Controlled polymer synthesis
Metal catalyzed cross-coupling polymerizations
The majority of conjugated polymers are synthesized via metal catalyzed cross-coupling reactions eg. Ni mediated reactions is shown below.
P3HT synthesis
P3HT synthesis was originally thought to occur via a step-growth polymerization.
When Ni(0) reductively eliminates, it can in theory reinsert into any Ar-Br bond. If this were to occur, this would be a step-growth polymerization
Externally initiated P3HT synthesis
TM = transmetallation RE = reductive elimination OA = oxidative addition
Restricted to only being able to use PPh3 as a ligand. dppp gives H/Br polymer.
Doubina and Luscombe, Macromolecules, 2009, 42, 7670
adapting to ligands other than PPh3 A novel method for the external initiated polymerizations of P3HT has developed by CMDITR researchers. The method produces a polymer with a well-defined molecular weight, narrow polydispersity index (PDI), 100% initiation efficiency, 100% regioregularity. This work represents the most control achieved for the synthesis of P3HT.
H. Bronstein, C. K. Luscombe, J. Am. Chem. Soc., 2009, 131, 12894
References
- ↑ J. E. Anthony; J. S. Brooks; D. L. Eaton; S. R. Parkin; J. Am. Chem. Soc. 2001, 123, 9482-9483.
- ↑ S. J. Park; C. C. Kuo; J. E. Anthony; T. N. Jackson; Tech. Dig. − Int. Electron Devices Meet. 2006, 113.
- ↑ Weidkamp, K. P.; Afzali, A.; Tromp, R. M.; and Hamers, R. J. J. Am. Chem. Soc., 2004, 126, 12740.
- ↑ Afzali, A.; Dimitrakopoulos, C. D.; Breen, T. L. J. Am. Chem. Soc., 2002, 124, 8812.
- ↑ A. J. Lovinger; H. E. Katz; A. Dodabalapur Chem. Mater., 1998, 10, 3275.
- ↑ A. R. Murphy; J. M. J. Fréchet; P. Chang; J. Lee; V. Subramanian J. Am. Chem. Soc., 2004, 126, 1596.
- ↑ Katz, H. E.; Lovinger, A. J.; Johnson, J.; Kloc, C.; Slegrist, T.; Li, W.; Lin, Y. Y.; Dodabalapur, A. Nature 2000, 404, 478
- ↑ F. Würthner; V. Stepanenko; Z. Chen; C. R. Saha-Möller; N. Kocher; D. Stalke J. Org. Chem. 2004, 69, 7933.
- ↑ Lacramioara M. Popescu, Patrick van 't Hof, Alexander B. Sieval, Harry T. Jonkman, and Jan C. Hummelen Appl. Phys. Lett. 89 213507 (2006)
- ↑ M.-H. Yoon; S. A. DiBenedetto; M. T. Russell; A. Facchetti; T. J. Marks Chem. Mater., 2007, 19, 4864–4881.