Difference between revisions of "The Polyene Series"
Jump to navigation
Jump to search
Cmditradmin (talk | contribs) |
Cmditradmin (talk | contribs) m |
||
| Line 1: | Line 1: | ||
[[Main_Page#Electronic Band Structure of Organic Materials|Return to Band Structure Menu]] | [[Main_Page#Electronic Band Structure of Organic Materials|Return to Band Structure Menu]] | ||
[[Bloch's Theorm| Next Topic]] | [[Bloch's Theorm| Next Topic]] | ||
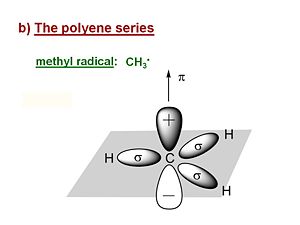
[[Image: | [[Image:methyl_radical.JPG|thumb|300px|The Methyl Radical]] | ||
Now we will explore the electronic properties of the polyenes; methylene, ethylene, butadiene etc. Its useful first to look at the methyl radical | Now we will explore the electronic properties of the polyenes; methylene, ethylene, butadiene etc. Its useful first to look at the methyl radical CH<sub>3</sub>ċ. It has a planar structure with the carbon and three of the four valence in the plane. The hydrogens are in sigma orbitals which are symmetrical about the plane of the bond. The unpaired electron or radical is in a 2pz atomic orbital that is perpendicular to the plane of the molecule. The pi AO for that radical has a lobe above the plane, and a negative lobe below the plane. The probability of the electron is always zero at the nucleus. The lobes in the diagram represent the surface within which there is an 80% probability of finding the electron. | ||
Revision as of 13:43, 20 May 2009
Return to Band Structure Menu Next Topic
Now we will explore the electronic properties of the polyenes; methylene, ethylene, butadiene etc. Its useful first to look at the methyl radical CH3ċ. It has a planar structure with the carbon and three of the four valence in the plane. The hydrogens are in sigma orbitals which are symmetrical about the plane of the bond. The unpaired electron or radical is in a 2pz atomic orbital that is perpendicular to the plane of the molecule. The pi AO for that radical has a lobe above the plane, and a negative lobe below the plane. The probability of the electron is always zero at the nucleus. The lobes in the diagram represent the surface within which there is an 80% probability of finding the electron.