Difference between revisions of "Light Emitting Electrochemical Processes"
| Line 46: | Line 46: | ||
<gallery widths=400 heights=300> | <gallery widths=400 heights=300> | ||
Image:Oled1_15_phosphor.png|Phosphorescence with linked systems | |||
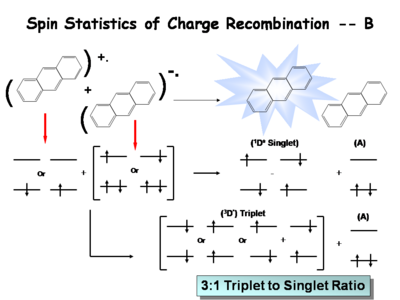
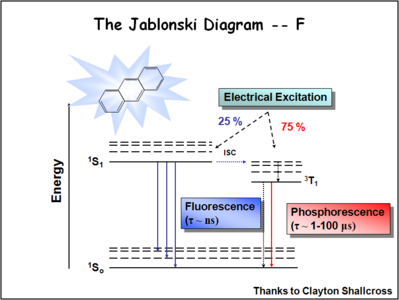
Image:Oled1_19_jablonski ratio.png|When see electrochemical excitation of these systems we tend to find that the number singlet state and triplet states partitions with 25% of energy deposited as singlet states and 75%of energy deposited in triplet states. This has a significant impact the optimization of organic light emitting diodes that either going to use fluorescent molecules to create the light to produce the light or phosphorescent dopants to create the light. The advent of the use of phosphorescent dopants has increased the efficiency to the near fluorescent lighting levels.Ratio of energy available. | |||
</gallery> | </gallery> | ||
Revision as of 15:31, 6 May 2009
Return to OLED Menu | Next Topic
This article serves as an introduction to the design and chemistry of organic light emitting diodes (OLEDs).
Light Emission from Recombination
Light emission in the OLED arises from recombination (electron transfer) reactions of the cation and anion radical of conjugated aromatic molecules. Several decades ago it was noted that poly(acenes) and related poly-aromatic hydrocarbons, in very dry nonaqueous (non-polar) solvents can be reduced by one electron (chemically or electrochemically) to produce an energetic radical anion state (D-). These same molecules can often be oxidized by one electron to produce a cation radical state (A+). Should A+ and D- encounter each other in solution, a “recombination” electron transfer reaction occurs. The excess free energy in this reaction can be deposited in one of the molecular species to form its lowest excited state (singlet), or in some cases, its lowest triplet excited state. These states are the same as those created by photoexcitation of the molecule. Emission from this state occurs with a lifetime of nanoseconds, with quantum yields approaching 100% in some cases. These “electrogenerated chemiluminescence” (ECL) processes are direct analogues of the charge recombination processes which occur in the condensed phase in an OLED. They are also closely related to the chemiluminescence and bioluminescence processes which occur in living organisms, the most easily recognized example being the luminescence processes which occur in fireflies.
It was quickly realized that in order to create the emissive state by injection charge the following processes took place:
<embed_document width=40% height=300 >http://depts.washington.edu/cmditr/images/OLEDredox.pdf</embed_document>
We can write this from the point of view of a homogenous electrochemical process. At the same time this was being done in the condensed phase people were beginning to explore this process in solution. Rudy Marcus used this as a central tenant in his development of electron transfer theory between small molecule systems.
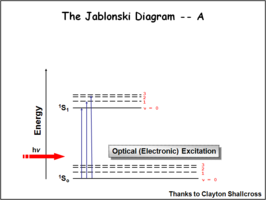
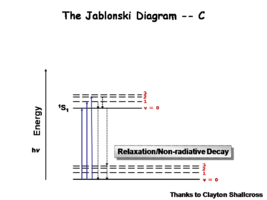
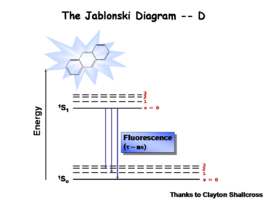
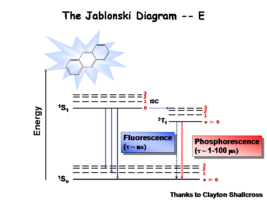
The Jablonski Diagram
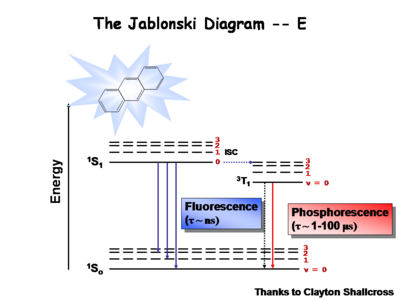
The Jablonksi diagram is a simple way to describe what is happening with small molecules and small conjugated aromatic systems. The Jablonski diagram is used to describe the energy (wavelength) of absorbance and luminescence for aromatic molecules.
Once the excited singlet state has been formed there is the possibility of intersystem crossing to a triplet state. This change in spin is a forbidden process (an energy transition not normally allowed by quantum mechanics)and as a consequence triplet states tend to be much longer-lived. This chart shows both the vibronic excited state and ground state.
Most molecules have lifetimes of 1-100 microseconds. Compounds that are most useful for OLEDs have lifetimes closer to 1 microsecond. Most people are familiar with molecules that phosphoresce with much longer lifetimes, and are therefore less useful for displays. These tend to have emission events at much longer wavelengths.
Color of Absorption and Emission
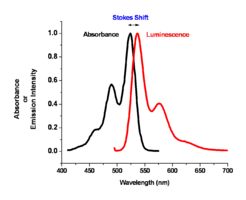
The color of absorption and emission in simple molecular systems is controlled by the structure of the molecule and by the degree of conjugation in the aromatic system. As the number of aromatic rings increases in these molecular systems, the energy for both the absorption and emission events goes down, shifting them to the red side of the spectrum. The same can be said for the carotenoid-like assemblies where increasing the number of double bonds in the system changes both the energy of absorption and emission. For most polyacine-like systems, there is an absorption and emission event, a small Stokes shift, and a change of wavelength of these two depending on the degree of conjugation in the aromatic system.
The Ratio of Singlet State to Triplet
The ratio of singlet state to triplet state formation helps to determine the OLED efficiency.
When see electrochemical excitation of these systems we tend to find that the number singlet state and triplet states partitions with 25% of energy deposited as singlet states and 75%of energy deposited in triplet states. This has a significant impact the optimization of organic light emitting diodes that either going to use fluorescent molecules to create the light to produce the light or phosphorescent dopants to create the light. The advent of the use of phosphorescent dopants has increased the efficiency to the near fluorescent lighting levels.Ratio of energy available.
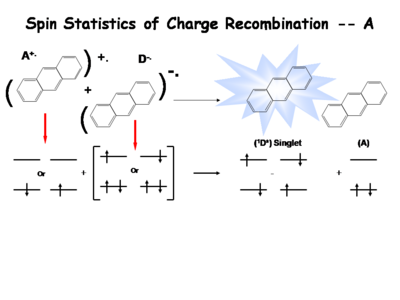
How does this happen? The spin statistics of recombination can be described in a number of ways. This is the simplest way but there are some short comings to this simplistic description. We start with a molecule in which an electron has been removed the spin of the remaining electron in the two level diagram can either be up or down. The electron in the donor molecular can either be spin up or spin down. During the process of electron transfer we create either an excited singlet state of the acceptor and the neutral donor or excited singlet state of the donor and the neutral acceptor.
The details of this are somewhat complicated. During this electron transfer event there forms a contact ion pair or solvent separated ion pair if it is in solution. It is single entity and by this means we can exchange electrons and create either a singlet of the donor or singlet of the acceptor. Typically it the molecule with the lowest emissive energy that winds up having the excess energy from this process. In this case you can’t really tell because they were both identical to begin with. In actual organic light emitting diode it is the lowest band gap molecule that ends up being the emissive species.
Finally, to form the triplet state you can have three types of triplets. Thus it is three times as likely to form a triplet as it is a singlet which accounts for the 3:1 triplet to singlet ratio in systems of this type. It is debated whether or not these ratios apply in the condensed phases. They apply in solutions and early indications are that it also applies in the condensed state. The consequence of this is that if you are going to optimize a light source you must harvest as much of energy of the triplet state as possible.
Electrogenerated Chemiluminescence Studies
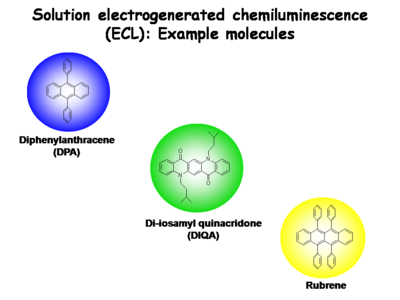
Small molecules are used in electrogenerated chemiluminescence (ECL) studies to help elucidate these light emitting processes in “condensed phases”
Several other molecular species are now known to provide for stable one-electron reduced states and one-electron oxidized states, in dry, non-polar solvents. Their recombination charge transfer processes produce blue-emitting states (diphenylanthracene, DPA), green-emitting states (di-isoamylquinacridone, DIQA (and other N,N’ dialkyl derivatives of quinacridone)), yellow emitting states (rubrene), and red-emitting states (ruthenium-trisbypryidine, Ru(bpy)+3). In all of these molecules their emissive states can be produced by charge transfer reactions between their reduced and oxidized forms. The separation in formal potentials for oxidation by one electron and reduction by one electron, expressed in electron volts, slightly exceeds the energy needed to directly excite the molecule to its lowest singlet state (S0 → S1).