Difference between revisions of "Changes in Absorption Spectra"
Cmditradmin (talk | contribs) m |
|||
| (2 intermediate revisions by the same user not shown) | |||
| Line 10: | Line 10: | ||
=== Terminology for absorption shifts === | === Terminology for absorption shifts === | ||
[[Image:Bathochromic.png|thumb|300px|]] | [[Image:Bathochromic.png|thumb|300px|Bathochromic, Hypsochromic, Hyperchromic, Hypochromic shifts summarized]] | ||
Changes in chemical structure or the environment lead to changes in the absorption spectrum of molecules and materials. There are several terms that are commonly used to describe these shifts, that you will see in the literature, and with which you should be familiar. | Changes in chemical structure or the environment lead to changes in the absorption spectrum of molecules and materials. There are several terms that are commonly used to describe these shifts, that you will see in the literature, and with which you should be familiar. | ||
| Line 24: | Line 24: | ||
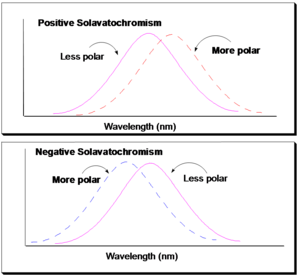
[[Image:Solvatocrhomism.png|thumb|300px|Negative and positive solvatochromism]] | [[Image:Solvatocrhomism.png|thumb|300px|Negative and positive solvatochromism]] | ||
If | If as substance shifts to a lower energy state with a longer wavelength, it is referred to as a Bathochromic shift or (also called) red shift. The color will move more toward the red. Conversely, something that moves to higher energy will be referred to as a hypsochromic shift. If there is an increase in the absorptivity or cause the spectrum to become more intense, it will be referred to as a hyperchromic shift. But a decrease is referred to as a hypochromic shift. There is a variety of factors that can cause these changes. One of the factors is found in a process known as solvatochromism. This explains why certain molecules can, in a profound way, look very different in terms of their color depending on whether the molecules are in a polar or non-polar solvent. | ||
Solvatochromism is the property of a molecule changing its color as a function of the solvent polarity. But it is actually more complex than that. It can be related to the solvent polarizability as well. Basically it is the change in the color of a material, or change in the spectrum, as a function of the dielectric properties of the solvent. The dielectric properties of the solvent have polarizability and polarity built into them. Therefore, if molecules go from a less polar solvent to a more polar solvent and a red shift or a bathochromic shift occurs, then the substance is referred to as being positively solvatochromic. Conversely if you put molecules into a more polar solvent and a blue shift occurs, i.e. higher energy, the molecules are referred to as being negatively solvatochromic. | Solvatochromism is the property of a molecule changing its color as a function of the solvent polarity. But it is actually more complex than that. It can be related to the solvent polarizability as well. Basically it is the change in the color of a material, or change in the spectrum, as a function of the dielectric properties of the solvent. The dielectric properties of the solvent have polarizability and polarity built into them. Therefore, if molecules go from a less polar solvent to a more polar solvent and a red shift or a bathochromic shift occurs, then the substance is referred to as being positively solvatochromic. Conversely if you put molecules into a more polar solvent and a blue shift occurs, i.e. higher energy, the molecules are referred to as being negatively solvatochromic. | ||
<br clear='all'> | <br clear='all'> | ||
[[category:absorption]] | |||
<table id="toc" style="width: 100%"> | <table id="toc" style="width: 100%"> | ||
<tr> | <tr> | ||
Latest revision as of 11:59, 29 December 2009
| Previous Topic | Return to Absorption and Emission Menu | Next Topic |
Terminology for absorption shifts
Changes in chemical structure or the environment lead to changes in the absorption spectrum of molecules and materials. There are several terms that are commonly used to describe these shifts, that you will see in the literature, and with which you should be familiar.
- Bathochromic: a shift of a band to lower energy or longer wavelength (often called a red shift).
- Hypsochromic: a shift of a band to higher energy or shorter wavelength (often called a blue shift).
- Hyperchromic: an increase in the molar absorptivity.
- Hypochromic: an decrease in the molar absorptivity.
Solvatochromism
If as substance shifts to a lower energy state with a longer wavelength, it is referred to as a Bathochromic shift or (also called) red shift. The color will move more toward the red. Conversely, something that moves to higher energy will be referred to as a hypsochromic shift. If there is an increase in the absorptivity or cause the spectrum to become more intense, it will be referred to as a hyperchromic shift. But a decrease is referred to as a hypochromic shift. There is a variety of factors that can cause these changes. One of the factors is found in a process known as solvatochromism. This explains why certain molecules can, in a profound way, look very different in terms of their color depending on whether the molecules are in a polar or non-polar solvent.
Solvatochromism is the property of a molecule changing its color as a function of the solvent polarity. But it is actually more complex than that. It can be related to the solvent polarizability as well. Basically it is the change in the color of a material, or change in the spectrum, as a function of the dielectric properties of the solvent. The dielectric properties of the solvent have polarizability and polarity built into them. Therefore, if molecules go from a less polar solvent to a more polar solvent and a red shift or a bathochromic shift occurs, then the substance is referred to as being positively solvatochromic. Conversely if you put molecules into a more polar solvent and a blue shift occurs, i.e. higher energy, the molecules are referred to as being negatively solvatochromic.
| Previous Topic | Return to Absorption and Emission Menu | Next Topic |