Difference between revisions of "Electromagnetic Radiation"
Cmditradmin (talk | contribs) (New page: Return to Luminescence Menu | Next Topic) |
Cmditradmin (talk | contribs) |
||
| Line 1: | Line 1: | ||
[[Main_Page#Luminescence and Color|Return to Luminescence Menu]] | | [[Main_Page#Luminescence and Color|Return to Luminescence Menu]] | | ||
[[Electromagnetic Spectrum|Next Topic]] | [[Electromagnetic Spectrum|Next Topic]] | ||
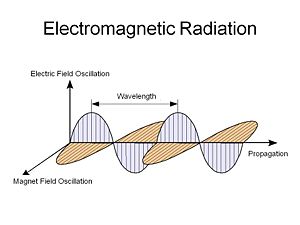
[[Image:Emwavepropagation.jpg|thumb|300px|]] | |||
Light is an electromagnetic radiation, an electric field that oscillates in both time and space along with a corresponding orthogonal magnetic field that oscillates with the same spatial and temporal periodicity. This was first described by Maxwell. | |||
When light gets into a material it interacts with the charged particles within the atom. Although both magnetic and electric field can be absorbed by materials, the interaction of the field of light is usually about 10^5 stronger than that of the magnetic field and thus to the first approximation we can concern ourselves only with the interaction of the electric field with matter. When the electric field of light gets into a material and it cause the electrons to move. | |||
Formally, the term “light” is strictly applicable to the visible portion of the electromagnetic spectrum, however we will use a looser definition that includes the entire visible and non-visible spectrum. | |||
Light behaves both as wave with properties of wavelength and frequency and as particle in that it is quantized in discrete packets known as photons. | |||
Wavelength lambda measure in nanometers (nm) | |||
Period T in seconds | |||
Frequency ν is 1/T measured in Hertz | |||
<math>c=\lambda \nuI\,\!</math> | |||
Revision as of 10:30, 4 May 2009
Return to Luminescence Menu | Next Topic
Light is an electromagnetic radiation, an electric field that oscillates in both time and space along with a corresponding orthogonal magnetic field that oscillates with the same spatial and temporal periodicity. This was first described by Maxwell.
When light gets into a material it interacts with the charged particles within the atom. Although both magnetic and electric field can be absorbed by materials, the interaction of the field of light is usually about 10^5 stronger than that of the magnetic field and thus to the first approximation we can concern ourselves only with the interaction of the electric field with matter. When the electric field of light gets into a material and it cause the electrons to move.
Formally, the term “light” is strictly applicable to the visible portion of the electromagnetic spectrum, however we will use a looser definition that includes the entire visible and non-visible spectrum.
Light behaves both as wave with properties of wavelength and frequency and as particle in that it is quantized in discrete packets known as photons. Wavelength lambda measure in nanometers (nm) Period T in seconds Frequency ν is 1/T measured in Hertz <math>c=\lambda \nuI\,\!</math>