Difference between revisions of "Fluorescent/Phosphorescent Dopants"
Cmditradmin (talk | contribs) |
Cmditradmin (talk | contribs) |
||
| (70 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
[[Main_Page# | <table id="toc" style="width: 100%"> | ||
[[Metal Complex Dopants|Next Topic]] | <tr> | ||
<td style="text-align: left; width: 33%">[[Organic Heterojunctions|Previous Topic]]</td> | |||
<td style="text-align: center; width: 33%">[[Main_Page#Organic_Light_Emitting_Diodes|Return to OLED Menu]]</td> | |||
<td style="text-align: right; width: 33%">[[Metal Complex Dopants|Next Topic]]</td> | |||
</tr> | |||
</table> | |||
==Example OLED applications== | ==Example OLED applications== | ||
The Pioneer car stereo was one of the first OLEDs to reach the market | The Pioneer car stereo was one of the first green and blue OLEDs to reach the market. | ||
Polymer OLEDs have an emissive layer of polyphenylenevinylene derivatives which are spin-cast or deposited with an inkjet printing process. The first polymer OLEDs consisted of two different polymer materials with different ionization potentials and electron affinities to create a heterojunction. These were capped with calcium or magnesium, and more recently with aluminum or other more stable metals. | |||
==Jablonski Diagram== | ==Jablonski Diagram== | ||
[[Image:Oled1 19 jablonski ratio.png|thumb|400px]] | [[Image:Oled1 19 jablonski ratio.png|thumb|400px]] | ||
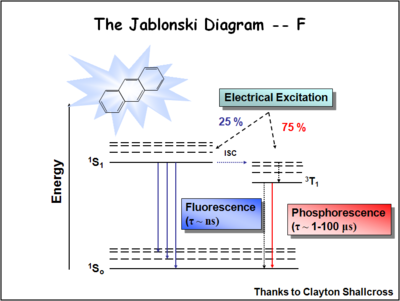
The Jablonski diagram shows a dopant molecule that can be excited from its singlet state to its first excited singlet state by overlap of its absorption with the emission from the host dye such as aluminum quinolate. The first dopants were primarily fluorescent dopants which shift the energy of the emission of the device slightly more to the red and improve the efficiency and stability of the device. The holy grail is | The Jablonski diagram shows a dopant molecule that can be excited from its singlet state to its first excited singlet state by overlap of its absorption with the emission from the host dye such as aluminum quinolate. The first dopants were primarily fluorescent dopants which shift the energy of the emission of the device slightly more to the red and improve the efficiency and stability of the device. The holy grail is to identify dopants which can capture the energy in the triplet state, which is 75% of the energy in the system. | ||
<br clear="all"> | <br clear="all"> | ||
==Device Efficiency== | ==Device Efficiency== | ||
[[Image:Device Efficiency.JPG|thumb|400px]] | [[Image:Device Efficiency.JPG|thumb|400px|External efficiency is a measure of how much light is produced given the power supplied.]] | ||
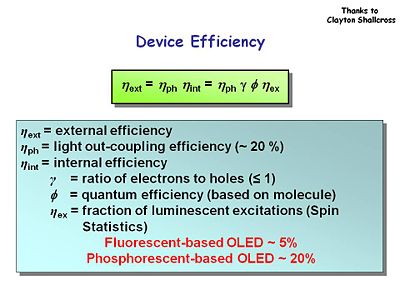
External power efficiency is a product of the light out-coupling efficiency and the internal efficiency. | External power efficiency is a product of the light out-coupling efficiency and the internal efficiency. Typical OLEDs only emit about 20% their light in the forward direction. The remaining energy is lost in substrate or waveguide modes which go to the side, which opens intriguing possibilities for sensors. Internal efficiency is related to the efficiency of all the components in the OLED. | ||
:<math>\eta _\mathrm{ext} = \eta_\mathrm{ph} \eta_\mathrm{int} = \eta_\mathrm{ph} \gamma \phi \eta_\mathrm{ex}\,\!</math> | |||
where | |||
:<math>\eta _\mathrm{ext}\,\!</math> = external efficiency | |||
:<math>\eta _\mathrm{ph}\,\!</math> = light out-coupling efficiency (~ 20 %) | |||
:<math>\eta _\mathrm{int}\,\!</math> = internal efficiency | |||
:<math>\gamma\,\!</math> = ratio of electrons to holes typically ≤ 1. There is an energy loss due this imbalance. | |||
:<math>\phi\,\!</math> = quantum efficiency of the emitting molecule. Optimal molecules will have the highest possible quantum efficiency for luminescence. | |||
<math> | :<math>\eta _\mathrm{ex}\,\!</math> = fraction of luminescent excitations based on spin statistics. Fluorescent OLEDs have external efficiency of about 5%, while phosphorescent OLEDs have up to 20% efficiency. | ||
The quantum efficiency can also be written | |||
:<math>\phi = \phi_R \cdot \phi_S \cdot \phi_F \cdot \phi_E\,\!</math> | |||
where | |||
:<math>\phi_R\,\!</math> is Langevin Recombination efficiency | |||
:<math>\phi_S\,\!</math> is Singlet Triplet Branching efficiency | |||
:<math>\phi_F\,\!</math> is Fluorescent efficiency | |||
:<math>\phi_R\,\!</math> is External Coupling efficiency | |||
== | ==Increasing Performance== | ||
You can increase device performance by balancing charge injection (γ). Single anthracene crystal devices had an efficiency < 1%. The first double-layer devices increased efficiency to 1%. The first doped devices used DCM2- a cumarin dye used in some dye lasers. It was doped at a high level of 10% works as a energy acceptor from the Alq<sub>3</sub> host and it boosted the external efficiency to 2.5%. It is an orange–red emitter which moves the emission from green to the red part of the spectrum. | |||
Tang et al. Appl. Phys. Lett. 1987, 51, 913-915 | See Tang <ref>Tang et al. Appl. Phys. Lett. 1987, 51, 913-915</ref> | ||
[[Image:Forstertransfer.JPG|thumb|400px|Förster is a non-radiative transfer of energy from a donor to an acceptor.]] | |||
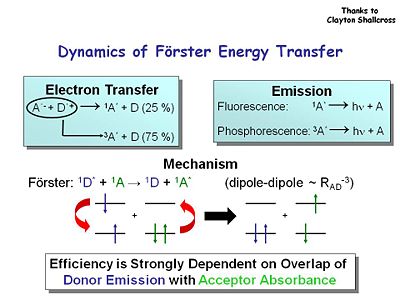
[[Image:Forstertransfer.JPG|thumb|400px]] | You can also increase device performance by increasing Φ using energy transfer. Consider the dynamics of Förster energy transfer. Spin statistics dictate that 75% of the electron transfer is in the triplet state. In the case of emission, the singlet state gives you fluorescence, and phosphorescence comes from the decay of the triplets state back to the ground state. It would be best to capture the energy from both states. Energy efficiency is strongly dependent on the overlap of donor emission with acceptor absorbance. | ||
You can also increase device performance by increasing & | |||
[[Image:Phosphorescent dopants.JPG|thumb|400px]] | See [[wikipedia:Fluorescence_resonance_energy_transfer]] | ||
<br clear="all"> | |||
[[Image:Phosphorescent dopants.JPG|thumb|400px|Phosphorescent doping increases the amount of intersystem crossing]] | |||
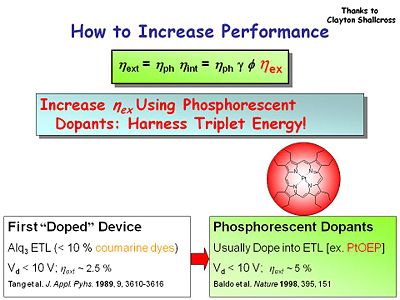
Because of the weak probability of intersystem crossing from fluorescent to the phosphorescent triplet state these molecules have lifetimes that are | Because of the weak probability of intersystem crossing from fluorescent to the phosphorescent triplet state these molecules have lifetimes that are milliseconds long. Display devices such as OLEDs need to have lifetimes that are shorter. | ||
Phosphorescent dopants such as the heavy metal porphyrin PtOEP | Phosphorescent dopants such as the heavy metal porphyrin PtOEP can increase efficiency to 5%. The heavy metal increases the probability of intersystem crossing. | ||
Baldo et al. Nature 1998, 395, 151 | See Baldo 1998 <ref>Baldo et al. Nature 1998, 395, 151</ref> | ||
The mechanism of triplet energy transfer | The mechanism of triplet energy transfer, called Dexter energy transfer, involves the exchange of an electron. This has a much closer distance-dependence than Forster transfer. The right phosphorescent dyes create triplet states at relatively low concentrations of these energy acceptors. Efficiency is strongly dependent on the orbital overlap between the donor and acceptor system. Heavy metal atoms such as platinum and iridium help to mix the singlet and triplet states by spin-orbit coupling. | ||
Tang et al. J. Appl. Phys. 1989, 9, 3610-3616 | See Tang 1989 <ref>Tang et al. J. Appl. Phys. 1989, 9, 3610-3616</ref> | ||
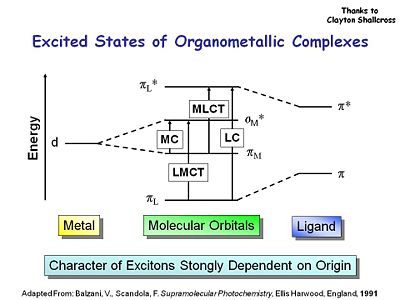
==Excited States of Organometallic Complexes== | ==Excited States of Organometallic Complexes== | ||
[[Image:organometalliccomplex.JPG|thumb|400px]] | [[Image:organometalliccomplex.JPG|thumb|400px|Ligand to Metal Charge Transfer (LMCT) compared to Metal to Ligand Charge Transfer (MLCT) with Metal Centered (MC) and Ligand Centered (LC)]] | ||
There are a couple of considerations when you work with organometallic complexes. There are ligand to metal transitions in the absorption of these molecules. There are ligand centered transitions, metal to ligand centered and | There are a couple of considerations when you work with organometallic complexes. There are ligand to metal transitions in the absorption of these molecules. There are ligand centered transitions, metal to ligand centered, and metal centered transitions. One must understand which of the molecular orbitals you are going to use to maximize the triplet state and the phosphorescence. | ||
See Balzani, V. and Scandola, F. Supramolecular Photochemistry, Ellis Harwood, England 1991, for a review of the rules for design of phosphorescent dopants. | See Balzani 1991 <ref>Balzani, V. and Scandola, F. Supramolecular Photochemistry, Ellis Harwood, England 1991</ref>, for a review of the rules for design of phosphorescent dopants. | ||
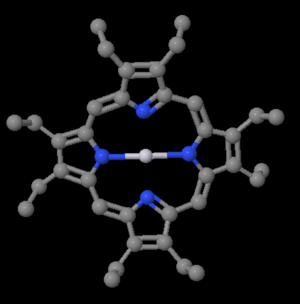
Platinum octoethyl porphyrin (Pt OEP) was the first phosphorescent dopant. The graph shows the host emission from an aluminum quinolate diode. At 20% PtOEP there is virtually no emission from the Alq<sub>3</sub> and nice red emission from the PtOEP. | |||
[[Image:PtOEP.png|thumb|300px|Platinum octaethylporphyrin|http://www.chemspider.com/ImageView.aspx?id=21169877&mode=3d]] | |||
<br clear="all"> | |||
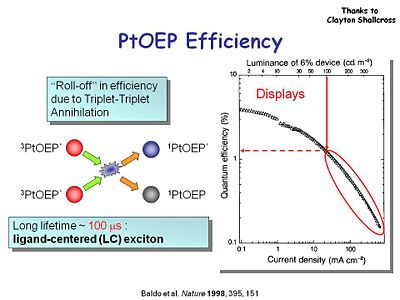
[[Image:OLED9_ptoep_efficiency.JPG|thumb|400px|Quantum efficiency drops off at higher current density.]] | |||
It is important to look at the quantum efficiency for these devices as a function of the applied current density. The optimal system will maximize light output while minimizing the current injected into the system. At a threshold luminance of 100 candelas per square meter (about the about the intensity of a computer screen display), PtOEP has a quantum efficiency of 1% and current density of 10 mA per square centimeter. This is not bad but could be better. PtOEP might not be the best choice for an emitter because of its long lifetime of 100 microseconds. Some energy will be lost through this phosphorescence because it lasts so long and there are other non-radiative decay processes that suck energy away. | |||
<br clear='all'> | |||
== References == | |||
<references/> | |||
[[category:organic LED]] | |||
[[category:luminescence]] | |||
<table id="toc" style="width: 100%"> | |||
<tr> | |||
<td style="text-align: left; width: 33%">[[Organic Heterojunctions|Previous Topic]]</td> | |||
<td style="text-align: center; width: 33%">[[Main_Page#Organic_Light_Emitting_Diodes|Return to OLED Menu]]</td> | |||
<td style="text-align: right; width: 33%">[[Metal Complex Dopants|Next Topic]]</td> | |||
</tr> | |||
</table> | |||
Latest revision as of 10:52, 9 August 2010
| Previous Topic | Return to OLED Menu | Next Topic |
Example OLED applications
The Pioneer car stereo was one of the first green and blue OLEDs to reach the market.
Polymer OLEDs have an emissive layer of polyphenylenevinylene derivatives which are spin-cast or deposited with an inkjet printing process. The first polymer OLEDs consisted of two different polymer materials with different ionization potentials and electron affinities to create a heterojunction. These were capped with calcium or magnesium, and more recently with aluminum or other more stable metals.
Jablonski Diagram
The Jablonski diagram shows a dopant molecule that can be excited from its singlet state to its first excited singlet state by overlap of its absorption with the emission from the host dye such as aluminum quinolate. The first dopants were primarily fluorescent dopants which shift the energy of the emission of the device slightly more to the red and improve the efficiency and stability of the device. The holy grail is to identify dopants which can capture the energy in the triplet state, which is 75% of the energy in the system.
Device Efficiency
External power efficiency is a product of the light out-coupling efficiency and the internal efficiency. Typical OLEDs only emit about 20% their light in the forward direction. The remaining energy is lost in substrate or waveguide modes which go to the side, which opens intriguing possibilities for sensors. Internal efficiency is related to the efficiency of all the components in the OLED.
- <math>\eta _\mathrm{ext} = \eta_\mathrm{ph} \eta_\mathrm{int} = \eta_\mathrm{ph} \gamma \phi \eta_\mathrm{ex}\,\!</math>
where
- <math>\eta _\mathrm{ext}\,\!</math> = external efficiency
- <math>\eta _\mathrm{ph}\,\!</math> = light out-coupling efficiency (~ 20 %)
- <math>\eta _\mathrm{int}\,\!</math> = internal efficiency
- <math>\gamma\,\!</math> = ratio of electrons to holes typically ≤ 1. There is an energy loss due this imbalance.
- <math>\phi\,\!</math> = quantum efficiency of the emitting molecule. Optimal molecules will have the highest possible quantum efficiency for luminescence.
- <math>\eta _\mathrm{ex}\,\!</math> = fraction of luminescent excitations based on spin statistics. Fluorescent OLEDs have external efficiency of about 5%, while phosphorescent OLEDs have up to 20% efficiency.
The quantum efficiency can also be written
- <math>\phi = \phi_R \cdot \phi_S \cdot \phi_F \cdot \phi_E\,\!</math>
where
- <math>\phi_R\,\!</math> is Langevin Recombination efficiency
- <math>\phi_S\,\!</math> is Singlet Triplet Branching efficiency
- <math>\phi_F\,\!</math> is Fluorescent efficiency
- <math>\phi_R\,\!</math> is External Coupling efficiency
Increasing Performance
You can increase device performance by balancing charge injection (γ). Single anthracene crystal devices had an efficiency < 1%. The first double-layer devices increased efficiency to 1%. The first doped devices used DCM2- a cumarin dye used in some dye lasers. It was doped at a high level of 10% works as a energy acceptor from the Alq3 host and it boosted the external efficiency to 2.5%. It is an orange–red emitter which moves the emission from green to the red part of the spectrum.
See Tang [1]
You can also increase device performance by increasing Φ using energy transfer. Consider the dynamics of Förster energy transfer. Spin statistics dictate that 75% of the electron transfer is in the triplet state. In the case of emission, the singlet state gives you fluorescence, and phosphorescence comes from the decay of the triplets state back to the ground state. It would be best to capture the energy from both states. Energy efficiency is strongly dependent on the overlap of donor emission with acceptor absorbance.
See wikipedia:Fluorescence_resonance_energy_transfer
Because of the weak probability of intersystem crossing from fluorescent to the phosphorescent triplet state these molecules have lifetimes that are milliseconds long. Display devices such as OLEDs need to have lifetimes that are shorter.
Phosphorescent dopants such as the heavy metal porphyrin PtOEP can increase efficiency to 5%. The heavy metal increases the probability of intersystem crossing.
See Baldo 1998 [2]
The mechanism of triplet energy transfer, called Dexter energy transfer, involves the exchange of an electron. This has a much closer distance-dependence than Forster transfer. The right phosphorescent dyes create triplet states at relatively low concentrations of these energy acceptors. Efficiency is strongly dependent on the orbital overlap between the donor and acceptor system. Heavy metal atoms such as platinum and iridium help to mix the singlet and triplet states by spin-orbit coupling.
See Tang 1989 [3]
Excited States of Organometallic Complexes
There are a couple of considerations when you work with organometallic complexes. There are ligand to metal transitions in the absorption of these molecules. There are ligand centered transitions, metal to ligand centered, and metal centered transitions. One must understand which of the molecular orbitals you are going to use to maximize the triplet state and the phosphorescence.
See Balzani 1991 [4], for a review of the rules for design of phosphorescent dopants.
Platinum octoethyl porphyrin (Pt OEP) was the first phosphorescent dopant. The graph shows the host emission from an aluminum quinolate diode. At 20% PtOEP there is virtually no emission from the Alq3 and nice red emission from the PtOEP.
It is important to look at the quantum efficiency for these devices as a function of the applied current density. The optimal system will maximize light output while minimizing the current injected into the system. At a threshold luminance of 100 candelas per square meter (about the about the intensity of a computer screen display), PtOEP has a quantum efficiency of 1% and current density of 10 mA per square centimeter. This is not bad but could be better. PtOEP might not be the best choice for an emitter because of its long lifetime of 100 microseconds. Some energy will be lost through this phosphorescence because it lasts so long and there are other non-radiative decay processes that suck energy away.
References
| Previous Topic | Return to OLED Menu | Next Topic |