Difference between revisions of "Electronegativity and Bonding Between Atoms"
Cmditradmin (talk | contribs) |
Cmditradmin (talk | contribs) m |
||
| (19 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
[[Main_Page#Molecular | <table id="toc" style="width: 100%"> | ||
[[ | <tr> | ||
<td style="text-align: left; width: 33%">[[Atomic Orbitals and Nodes|Previous Topic]]</td> | |||
<td style="text-align: center; width: 33%">[[Main_Page#Molecular Orbitals|Return to Molecular Orbitals Menu]]</td> | |||
<td style="text-align: right; width: 33%">[[sigma and pi Orbitals|Next Topic]]</td> | |||
</tr> | |||
</table> | |||
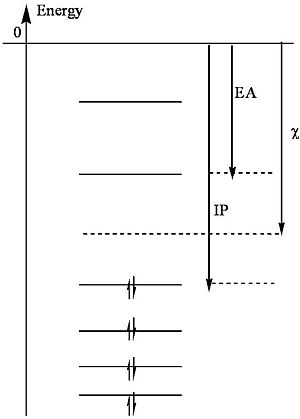
<math>\chi = \frac{IP + EA}{ 2}\,\!</math> | |||
[[Image:Ionization_pot.jpg|thumb|300px|Energy level diagram showing the relation between electronegativity (χ), electron affinity (EA), ionization potential (IP) to the vacuum level and the valence band]] | |||
Electronegativity describes how much an atom hold its electrons tightly. An atom that is less electronegative will be more likely to give up its electrons. The electronegativity of atoms determines the degree that electrons are transferred between molecules or shared between bonding atoms. | |||
Mulliken presents a useful definition of electronegativity (χ) as the average of the ionization potential (IP) and the electron affinity (EA). | |||
:<math>\chi = \frac{IP + EA}{ 2}\,\!</math> | |||
Note that the ionization potential is the energy required to remove an electron from an atom (to vacuum) and the electron affinity is the energy released when an atom captures an electron (into the lowest unfilled orbital). | Note that the ionization potential is the energy required to remove an electron from an atom (to vacuum) and the electron affinity is the energy released when an atom captures an electron (into the lowest unfilled orbital). | ||
The more electronegative the atom the lower the energy of the orbital within a given row. | The more electronegative the atom is the lower the energy of the orbital within a given row. Moving to the right on the periodic table across a row the electronegativity increases because the number of protons increases which increases the pull on the electrons. When the difference in electronegativity is great, as in Na<sup>+</sup> and Cl<sup>-</sup>, the electron is transferred from the electropositive species to the more electronegative species. These ionic compounds are held together by electrostatic forces and form a salt. In pure covalent bonds between like atoms each of the bonding electrons are shared equally. The electron density is highest between the atoms an there is no dipole moment. When there is slight difference in electronegativities for example in in C-F bond, the fluorine holds the electrons closer, the carbon less so. The whole bond has a polarity with unequal distribution of electrons.. | ||
Here is a summary of the different kinds of bonds. | Here is a summary of the different kinds of bonds. | ||
{| | {|border="1" | ||
|- | |- | ||
! Ionic Bonding | ! Ionic Bonding | ||
| Line 42: | Line 50: | ||
|} | |} | ||
[[category:chemical bonding]] | |||
[[category:molecular orbitals]] | |||
<br clear='all'> | |||
<table id="toc" style="width: 100%"> | |||
<tr> | |||
<td style="text-align: left; width: 33%">[[Atomic Orbitals and Nodes|Previous Topic]]</td> | |||
<td style="text-align: center; width: 33%">[[Main_Page#Molecular Orbitals|Return to Molecular Orbitals Menu]]</td> | |||
<td style="text-align: right; width: 33%">[[sigma and pi Orbitals|Next Topic]]</td> | |||
</tr> | |||
</table> | |||
Latest revision as of 12:27, 29 December 2009
| Previous Topic | Return to Molecular Orbitals Menu | Next Topic |
Electronegativity describes how much an atom hold its electrons tightly. An atom that is less electronegative will be more likely to give up its electrons. The electronegativity of atoms determines the degree that electrons are transferred between molecules or shared between bonding atoms.
Mulliken presents a useful definition of electronegativity (χ) as the average of the ionization potential (IP) and the electron affinity (EA).
- <math>\chi = \frac{IP + EA}{ 2}\,\!</math>
Note that the ionization potential is the energy required to remove an electron from an atom (to vacuum) and the electron affinity is the energy released when an atom captures an electron (into the lowest unfilled orbital).
The more electronegative the atom is the lower the energy of the orbital within a given row. Moving to the right on the periodic table across a row the electronegativity increases because the number of protons increases which increases the pull on the electrons. When the difference in electronegativity is great, as in Na+ and Cl-, the electron is transferred from the electropositive species to the more electronegative species. These ionic compounds are held together by electrostatic forces and form a salt. In pure covalent bonds between like atoms each of the bonding electrons are shared equally. The electron density is highest between the atoms an there is no dipole moment. When there is slight difference in electronegativities for example in in C-F bond, the fluorine holds the electrons closer, the carbon less so. The whole bond has a polarity with unequal distribution of electrons..
Here is a summary of the different kinds of bonds.
| Ionic Bonding | Polar Covalent Bonding | Covalent Bonding |
|---|---|---|
| Forms salts that dissociated in water | Forms bonds which have dipole | Forms bonds with no dipole |
| Between atoms of the far left and far right of the periodic table | Betwen atoms of different columns usually on the right side of the table | Between two like atoms |
| Large differences in electronegativities | Moderate differences in electronegativities | No difference in electronegativities |
| Very unequal sharing, electron is transferred form one atom to another | Unequal sharing of electrons | Equal sharing of electrons |
| Na+Cl- | C-F bond | F-F bond
|
| Previous Topic | Return to Molecular Orbitals Menu | Next Topic |