Difference between revisions of "Self Assembled Materials"
Cmditradmin (talk | contribs) |
Cmditradmin (talk | contribs) |
||
| Line 22: | Line 22: | ||
The work function of a metal is very sensitive to the presence of any adsorbates present on the surface. Experiments have revealed that even physisorbed atoms of inert gases such as argon or xenon influence the work function on a variety of metallic substrates.<sup>11-13</sup> Even though there is no significant charge redistribution in the inert-gas atoms near the metal surface, due to a chemical reaction, there is a substantial charge redistribution on the surface after the physisorption has taken place, which changes the surface dipole on the surface. Two major physical phenomena are responsible for this. First, the electronic density extending outside of the metal surface is pushed back by the repulsive force of the electronic cloud of the inert gas atoms; this is referred to as Pauli push-back, or sometimes as the “pillow effect”.<sup>3,14</sup> Second, van der Waals interactions between the inert-gas atom and the metal surface cause polarization of the otherwise highly symmetric electronic cloud of the inert-gas atom. | The work function of a metal is very sensitive to the presence of any adsorbates present on the surface. Experiments have revealed that even physisorbed atoms of inert gases such as argon or xenon influence the work function on a variety of metallic substrates.<sup>11-13</sup> Even though there is no significant charge redistribution in the inert-gas atoms near the metal surface, due to a chemical reaction, there is a substantial charge redistribution on the surface after the physisorption has taken place, which changes the surface dipole on the surface. Two major physical phenomena are responsible for this. First, the electronic density extending outside of the metal surface is pushed back by the repulsive force of the electronic cloud of the inert gas atoms; this is referred to as Pauli push-back, or sometimes as the “pillow effect”.<sup>3,14</sup> Second, van der Waals interactions between the inert-gas atom and the metal surface cause polarization of the otherwise highly symmetric electronic cloud of the inert-gas atom.The two described mechanisms provide an oversimplified picture of the charge-redistribution process. Contribution from orbital interactions is also invoked as another mechanism of charge redistribution. See ref. 24.''' | ||
This results in the formation of a sheet of dipoles in the space occupied by the adsorbed atoms which alters the potential landscape at the vacuum / metal interface.<sup>12,13'''</sup> | |||
On the basis of simple electrostatic considerations one can calculate the dipole-moment surface density corresponding to the measured change of the work function.<sup>3</sup> The work function change, ''??<sub>m''</sub>, caused by a sheet of dipoles residing on the surface is expressed by the Helmholtz equation:<sup>3,15</sup> | On the basis of simple electrostatic considerations one can calculate the dipole-moment surface density corresponding to the measured change of the work function.<sup>3</sup> The work function change, ''??<sub>m''</sub>, caused by a sheet of dipoles residing on the surface is expressed by the Helmholtz equation:<sup>3,15</sup> | ||
Revision as of 11:46, 23 September 2009
Influence of organic thiols on the work function of coinage metals
A critical factor in self assembled materials concerns the Work Function of Metals.
Work Function of Metals – Implications for Organic Electronics Applications
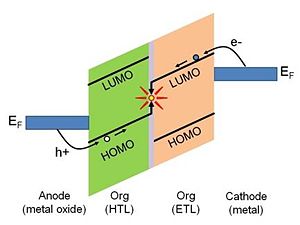
The presence of the surface dipole on the metal surface has important implications for electronics applications. Charge-carrier injection from a metal electrode to an active layer in a variety of optoelectronic devices is considered to be one of the crucial processes essential to the overall device performance.[1].[2] The energetics of the charge-injection process are defined by the relative positions of the Fermi level of the metal and the accessible energy levels of the organic active layer. This is depicted in Figure 3 for the case of a hypothetical simplified organic electroluminescent (EL) device.2 Consider the right-hand side of the energy diagram. In order for an electron to undergo a transfer from the metal cathode to the organic layer it must be supplied with sufficient energy to overcome the barrier existing at the interface. The electron-injection barrier, Δe, is defined as the energy difference between the work function of the metal and the solid-state electron affinity, EA, of the organic layer: Δe = φm - EA. The solid-state electron affinity and solid-state ionization potential discussed here are not the same as the corresponding values measured for bulk organic layer. The contact with the metal electrode causes so-called “band bending”, which influences the values of both electron affinity and ionization potential. This is discussed in more detail in Ref [3]
In the case of an EL device the energy needed to overcome the electron-injection barrier is supplied by applying a voltage between the electrodes. From the technological perspective the applied voltage must satisfy certain requirements, for example fall in a range that minimizes the power loss of the device, and allows for integration of the device into a standardized circuit. Thus, matching the solid-state electron affinity and solid-state ionization potential of the organic layers with the work functions of the metal electrodes is the key to obtaining a suitable charge injection during device operation.
The EL device described above is only one of the examples in which the metal electrode work function plays a crucial role in the overall device performance. Matching the work function of the electrodes with the organic-layer energy levels in order to balance the charge-carrier-injection barriers is very important in organic field-effect transistors (OFETs), photovoltaic devices, or organic light-emitting transistors (OLETs).3,7,8 Traditionally this has been addressed by choosing metals with low work function for the cathode, high-work-function materials for the anode, and matching the organic layer energy levels appropriately.3 Lately however, there has been a substantial effort to employ metal electrodes whose work function has been tuned by adsorbates.9,10 This approach is, in principle, more versatile than the use of different, often reactive metals, as it opens the possibility of using relatively chemically stable metals (such as coinage metals) with a layer of work-function-tuning adsorbate as electrodes in organic electronic applications.
Work Function of Metals – Influence of Adsorbates
The work function of a metal is very sensitive to the presence of any adsorbates present on the surface. Experiments have revealed that even physisorbed atoms of inert gases such as argon or xenon influence the work function on a variety of metallic substrates.11-13 Even though there is no significant charge redistribution in the inert-gas atoms near the metal surface, due to a chemical reaction, there is a substantial charge redistribution on the surface after the physisorption has taken place, which changes the surface dipole on the surface. Two major physical phenomena are responsible for this. First, the electronic density extending outside of the metal surface is pushed back by the repulsive force of the electronic cloud of the inert gas atoms; this is referred to as Pauli push-back, or sometimes as the “pillow effect”.3,14 Second, van der Waals interactions between the inert-gas atom and the metal surface cause polarization of the otherwise highly symmetric electronic cloud of the inert-gas atom.The two described mechanisms provide an oversimplified picture of the charge-redistribution process. Contribution from orbital interactions is also invoked as another mechanism of charge redistribution. See ref. 24.
This results in the formation of a sheet of dipoles in the space occupied by the adsorbed atoms which alters the potential landscape at the vacuum / metal interface.12,13
On the basis of simple electrostatic considerations one can calculate the dipole-moment surface density corresponding to the measured change of the work function.3 The work function change, ??m, caused by a sheet of dipoles residing on the surface is expressed by the Helmholtz equation:3,15
- <math>\Delta \phi_m = \frac {e \mu} {A \varepsilon \varepsilon_0}\,\!</math> Equation 3
where e is the elementary charge, ? is the dipole moment in the direction of the surface normal, is the area of the metal surface, ? is the dielectric constant of the dipole layer, and ?0 is the vacuum permittivity. Table 2 shows experimentally measured values of the work-function changes for a series of inert-gas / metal-surface systems together with the dipole-moment surface density induced by the inert-gas atoms calculated according to Equation 3.13 It can be seen that the adsorption of inert-gas atoms can lower the work function of coinage-metal surfaces by as much as 0.62 eV, which in the case of Cu(111) surface corresponds to ca. 13% drop in the work function upon adsorption of xenon. The dipole-moment surface densities calculated according to Equation 3 from the measured work-function changes in Table 2 are on the order of 1.5 D / nm2. This corresponds roughly to a separation of a whole elementary charge by 1 Å in each 3 nm2 of the surface.
The changes in the work function due physisorption of inert-gas atoms are interesting from the perspective of fundamental understanding of the electronic processes at the surfaces. However, inert gases do not form robust layers upon adsorption and they do not allow for much control of the dipole moment present on the surface after adsorption. The possibility of such control via synthetic design was opened with the development of the field of self-assembled monolayers (SAMs) of organic thiols on metals.
Table 2. Experimentally measured changes in the work function, ??m, of coinage metals upon adsorption of inert gases.* Values adapted from ref. 13. The dipole-moment surface densities calculated according to Equation 3 are given in the third column.
* The change in the work function, Δ φm, is defined as the difference in the work function of the clean substrate and the work function of the surface after the adsorption of inert gas atoms.
Self-Assembled Monolayers of Organic Thiols on Metals
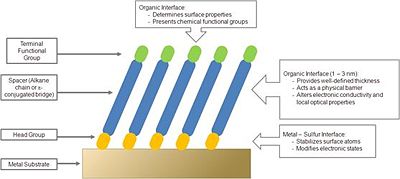
Self-Assembled Monolayers of organic thiols on metals have received considerable attention over the last two decades.16-19 From a purely scientific standpoint these structurally ordered systems offer a great opportunity for studying structure–property relationships of organic-inorganic interfaces. Studies of the influence of the molecular structure on the packing of thiols on noble-metal surfaces have led to important insights into the molecular scale morphology of the monolayers. It is now understood that the thiol group binds to gold and often long-range order is observed in the resulting organic adlayer.17-23 This understanding has further led to studies of the influence of synthetically accessible adsorbate structures on a variety of surface characteristics including wetting properties,20,24 electronic characteristics,25-27 and chemical reactivity.28-30 Figure 4 shows a schematic of a structure of an organic thiolate SAM on a metal surface, together with design motifs that can be used to tune the properties of the surface.
As discussed earlier, the work function of metals is affected by adsorbates present on the surface. The knowledge of adsorption characteristics of organic thiols on metals and the synthetic accessibility of a variety of structures makes these SAMs excellent systems to be employed in the systematic study of the influence of adsorbates on the work function of the metallic substrates.
Self-Assembled Monolayers of Alkanethiols and their Influence on the Work Function of Metals
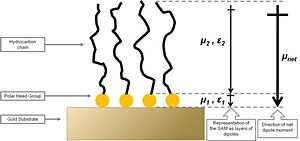
The first study addressing the effects of organic thiol SAMs on the work function of gold was published by Evans and Ulman.31 The authors performed ellipsometry and Kelvin-probe measurements on a series of alkanethiol SAMs with varying alkyl spacer lengths on a gold surface. Ellipsometry revealed that the thickness of the monolayers systematically increased with the alkyl chain length, which supported the formation of dense thiolate-bound monolayers on gold. The studied alkanethiol SAMs showed a linear decrease of the work function of the metal with the number of methylene units in the alkyl chain length on the monolayer constituent, with a slope of -9.3 meV / methylene group. This has been interpreted using a model involving a dipole layer residing on top of the metal, as depicted in Figure 5. The net dipole moment of the organic SAMs was found to have the positive end at the organic / air interface, thus effectively decreasing the work function as compared to clean gold (see Equation 3). The addition of methylene units in the alkyl chain increased the magnitude of the dipole moment showing the abovementioned trend in the work-function change with alkyl chain length. Extrapolating the measured changes of the work function to a hypothetical monolayer without methylene groups revealed that Au – S layer decreases the work function of the substrate by as much as ca. 0.5 eV, which is qualitatively consistent with charge rearrangement due to the formation of a chemical bond. Additionally, the dielectric constant of the alkyl chain layer, ?2 in Figure 5, was suggested to change with the alkyl chain length thus highlighting that both the SAM dipole moment and the dielectric constant influence the metal work function, the latter parameter because it effectively depolarizes the dipole layer. The decrease of the gold work function caused by the alkanethiols studied by Evans and Ulman was as large as 0.70 eV. However, it should be stressed that the method applied to measure the work function did not take into account any adsorbates that might have been present on the reference (in this case a “clean” gold surface); thus, the absolute value of the work function depression with respect to truly clean gold surface was most likely underestimated.
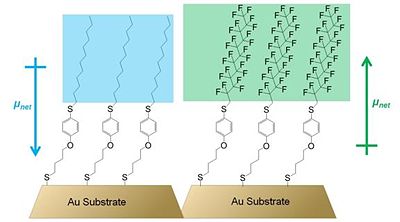
Further investigations of alkyl thiol monolayers containing a polar aromatic group showed that both the direction and the magnitude of the dipole moment of the molecules forming the monolayer are important factors determining the work function of the underlying metal. Evans et al. used Kelvin probe measurements to show that the structure of the organic adsorbate had a critical effect on the magnitude and the sign of the work-function change.32 In particular, the substitution of the terminal alkyl chain in one of the studied SAMs to a fluoroalkyl chain resulted in a net dipole moment of opposite direction to that of the SAM with the terminal alkyl chain (see Figure 6). In effect, while the terminal-alkyl-chain SAM causes a depression of the work function of gold by ca. 0.45 eV, the SAM with terminal fluoroalkyl chain increases the work function by as much as 0.75 eV. Thus, the authors clearly showed that the structure of the organic SAM, and in particular the effective net dipole moment of the organic structure, has a dramatic effect on the work-function change of the underlying metal.
Lu et al. successfully used Kelvin-probe force microscopy (KPFM) to image a gold surface patterned with a mixed alkylthiolate monolayer.33 Alkylthiol terminated with a methyl group and another alkylthiol terminated with a carboxylic acid group were patterned via the microcontact printing technique.19 The observed contrast in the KPFM images was an effect of the difference in the work function of the gold-surface regions coated with the two adsorbates possessing different dipole moments. In the case of the two different alkyl thiolates studied by Lü the contrast was as large as 0.4 eV. Furthermore, a gold surface patterned with methyl-group-terminated alkylthiols containing different numbers of methylene units showed a trend in the measured surface potential, corresponding to a linear decrease of the work function with the slope of ca. -14 meV / methylene group, a behavior qualitatively similar to that reported by Evans.31
In an elegant and comprehensive study Alloway and coworkers investigated the influence of alkanethiol and partially-fluorinated-alkanethiol SAMs on the work function of gold.34 In contrast to the reports Evans and Lu, the work of Alloway et al. was based on ultraviolet photoelectron spectroscopy (UPS), which is an absolute method performed under ultrahigh vacuum conditions, and thus it is anticipated to reflect the true values of the work function changes caused by thiolate SAMs on gold substrates. (As mentioned in section 1.4 any adsorbate will change the work function of a metallic surface. Thus, only working under ultrahigh vacuum conditions with properly cleaned metal surfaces can yield true work function values of the metal surface)
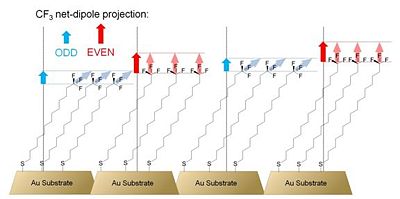
All of the samples of alkylthiolate SAMs on gold showed a work function more than 1 eV lower than the work function of clean gold. Similarly to previously mentioned reports,31,33 the authors showed that a change in the number of methylene units results in a change in the work function. Fitting the data points with a linear function yielded the slope of -19 meV / methylene unit, an absolute value larger than the values reported by both Evans et al. and Lu et al. Partially fluorinated alkyl thiolate SAMs showed an increase in the work function of the underlying substrate by as much as ca. 0.5 eV, consistent with the different direction of the dipole moment of the surface-attached molecules in the case of fluorinated and non-fluorinated chains. Analysis of the measured changes in the work function of gold as a function of the calculated projection of the molecular dipole moment onto the surface normal in the alkyl- and perfluoroalkylthiolate SAMs revealed that the Au – sulfur interaction depresses the work function by ca. 0.5 eV, which is the same value as that found by Evans et al. for similar systems.31 An interesting behavior was observed in a series of SAMs formed with alkylthiolates of different length terminated with a trifluoromethyl group. Figure 7 shows a schematic representation of these systems.
Due to the different orientation of the polar CF3 groups with respect to the surface normal in odd- and even-numbered alkylthiolate SAMs, the magnitude of the effective-dipole-moment projection onto the surface normal, shown in Figure 7 with blue and red arrows, is different and manifests itself with an odd-even effect on the measured work function of the underlying gold substrate.
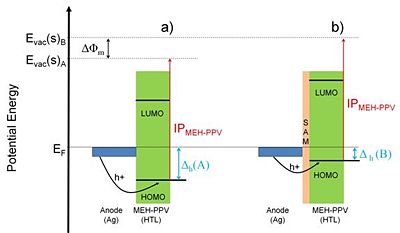
De Boer et al. studied the effects of alkylthiolate and perfluoroalkylthiolate SAMs on the work function of gold and silver.10 Using the Kelvin probe technique the researchers found qualitatively similar changes of the work function, caused by the studied SAMs, for both metals. In particular, the perfluorinated alkylthiolate SAMs increased the work function by 0.6 eV and 1.1 eV for gold and silver, respectively. The alkylthiolate SAMs, on the other hand, decreased the work function for both metals respectively by 0.8 eV, and 0.6 eV. Again, the changes in the work function caused by the organic monolayers were rationalized in terms of the SAM dipole layer and the resulting surface potential difference for different molecular structures. De Boer et al. took one step further and demonstrated the effect of the SAMs on the performance of organic diodes employing silver electrodes. These devices were built as models for organic light emitting diodes (OLEDs) based on the use of a semitransparent silver anode. The ionization potential of the active organic layer used in these OLEDs – poly[2-methoxy-5-(2'-ethyl-hexyloxy)-1,4-phenylene vinylene] (MEH-PPV) – is not matched with the untreated anode’s work function, resulting in a hole injection barrier of ca. 0.9 eV, which is a rather large value requiring operation of the device at high voltage. The devices described by the authors were prepared in a few configurations: a) Ag anode / MEH-PPV / Ag, b) Ag anode / perfluoroalkylthiolate SAM / MEH-PPV / Ag, and c) Ag anode / alkylthiolate SAM / MEH-PPV / Ag. Figure 8 shows the energy diagram of the Ag anode / organic layer interface for configurations a) and b). Due to the dipole layer of the perfluoroalkylthiolate SAM the work function of the silver anode surface was increased by 1.1 eV, thus effectively eliminating the hole-injection barrier, ?h, from the electrode to the organic layer and forming an ohmic contact. Indeed, the current – voltage characteristics for the investigated devices showed ohmic behavior in the device incorporating perfluoroalkylthiolate SAM, in contrast to the device without the SAM on top of the silver anode, which showed an onset voltage for the conduction. Interestingly, the presence of alkylthiolate SAM on the silver anode (configuration c) of the device) increased the onset voltage of the diode even further when compared to the device without any SAM. This was rationalized in terms of the reduction of the work function of silver by the alkylthiolate SAM, which resulted in the increase of the hole-injection barrier with respect to case a).
In summary, alkylthiolate-based monolayers on coinage metals have been shown to reproducibly modify the work function of the underlying substrates. Additionally, the molecular structure of constituents of SAMs, together with simple electrostatic considerations, has been shown to be useful in terms of designing surfaces with a desired work-function change. It is important to stress that the modification of the work function of metal electrodes with alkylthiolate SAMs has been demonstrated independently by different research groups, and that the ability to tune the work function has been successfully applied to solve specific technological problems.9,10
Self-Assembled Monolayers of Conjugated Thiols and their Influence on the Work Function of Metals
A limitation on the use of monolayers consisting of long-chain alkyl thiols for modifying injection barriers between metals and organics is the intrinsic electrically insulating characteristics of the alkyl chains. Monolayers consisting of ?-conjugated thiols, which have been shown to exhibit considerably enhanced conductivity,26,35 may be more promising for this type of application. Monolayers composed of π-conjugated systems based on oligo(phenylethynyl)benzenethiols and oligo(phenyl)benzenethiols have been shown to form self-assembled domains on gold.21,22,36-38 A variety of data from different measurement techniques supports the formation of these SAMs on gold and silver with the thiol group binding to the underlying metal and the molecular backbone orienting itself nearly perpendicularly to the surface plane of the substrate.21,22,36,39-41 Even in the case of molecular structures based on biphenyl thiols with very polar substituents this structural arrangement seems to hold, as demonstrated by Kang et al. in their comprehensive report.22 As with alkylthiolate SAMs, the understanding of the structure of ?-conjugated thiolate SAMs on metal substrates opened the possibility to study the influence of the molecular structure on the electronic properties of the underlying substrates.
Campbell et al. studied the effects of oligo(phenylethynyl)benzenethiolate SAMs on copper on the performance of organic diodes.42 Two molecular systems were compared, with the substitution of a terminal hydrogen atom for a fluorine atom as the only difference between the SAM constituents. The researchers compared the current – voltage characteristics of organic diodes with different structures – Cu anode / MEH-PPV / Ca, and Cu anode / SAM / MEH-PPV / Ca. The onset voltage for the conduction in the studied devices showed the expected trend, with the lowest onset recorded for the SAM composed of the fluorine-substituted molecules and the highest onset voltage for the corresponding SAM without fluorine substitution. The rationalization of the observed effect invoked the difference in the hole-injection barrier from the electrode to the organic layer due to the change in the work function caused by the formation of SAMs. Kelvin probe measurements indeed showed that the work function of the copper electrodes coated with the different SAMs qualitatively follows the prediction based on the well known effect of the sheet of dipole moments on top of the metal.
Zehner et al. were the first to systematically study the work function modification of gold by conjugated thiols.43 The authors applied Kelvin-probe measurements to a series of oligo(phenylethynyl)benzenethiolate SAMs on gold. The rather impressive number of studied systems included molecular structures with different lengths of the ?-conjugated backbones as well as different polar end-substituents. The trends of the work-function change reported in this work are actually not consistent with the predictions based on the molecular dipole moment. In particular, the molecular systems with dipole moments that should theoretically lower the work function actually increase it. Nevertheless, the authors attributed the observed work-function changes to the different dipole moment values of the studied molecular systems, and further conclusions followed.
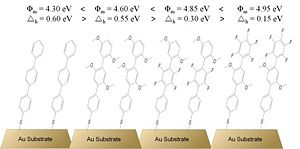
Chen et al. investigated work function of gold substrates coated with a series of differently substituted terphenyl thiolate SAMs.44 All of the studied systems showed a depression of the work function when compared with clean gold. A gold surface with an overlayer of a SAM of terphenylthiolate, characterized by only a small dipole moment, showed a work-function value 0.90 eV lower than that measured for the clean gold. Because the dipole moment of the molecule is small, this change has to be attributed to the charge redistribution caused by the formation of Au – S bond. Substitution of hydrogen on phenyl rings in the terphenylbackbone with fluorine atoms showed an increase of the work function of the underlying substrate, in accordance with qualitative predictions based on the dipole moment considerations. The authors showed their ability to tune the work function from 4.30 eV to 4.95 eV by simply using different SAMs on top of the gold electrodes. The structures of the SAMs and the corresponding values of the measured work function are shown in Figure 9. Further, the authors showed changes in the hole-injection barrier, Δh, into a copper-phthalocyanine (CuPc) layer deposited on top of the different SAM-coated gold substrates. This was done by measuring the position of the highest molecular energy level of CuPc (HOMO) with respect to the Fermi level for the different samples. The changes in the measured hole-injection barrier roughly follow the dependence: Δh ∝ φm, where φm is the work function measured for the SAM-coated gold substrates. Thus, the use of the different π-conjugated-thiolate SAMs allowed for tuning the hole-injection barrier from the gold electrode into CuPc organic layer. The values of the hole-injection barrier, Δh, are also shown in Figure 9.
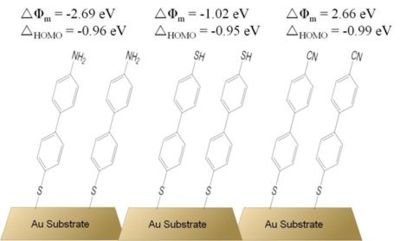
Apart from other experimental reports on the metal-work-function changes induced by ?-conjugated SAMs,45,46 particular attention should be brought to theoretical work dealing with the energy level alignment of organic overlayers on gold. Among those, the reports by Heimel et al.,3,47 and Romaner et al.15,48 are perhaps of the highest interest in the context of this thesis. These studies focus on theoretical description of biphenylthiolate-based systems as there is a vast amount of data showing these SAMs possess two dimensional order and their structure has been studied extensively, thus making them good model systems for theoretical investigations.19,22 Employing density functional theory (DFT), Heimel et al. showed that biphenylthiolate SAMs terminated with three different end-groups (a weak ?-donor –SH, a strong ?-donor –NH2, and a strong ?-acceptor –CN; see Figure 10) can result in large changes of the work function of the underlying gold substrate.3,47
Because of the electron-donating ability of the amino group to the ?-conjugated biphenyl backbone, this system exhibits a dipole moment with the positive end at the organic / vacuum interface, thus decreasing the work function of the substrate by 2.7 eV. On the other hand, a strongly electron-withdrawing substituent, the cyano group, forms a dipole with its positive end at the metal / organic interface. This results in an increased work function of the substrate by as much as 2.7 eV. Interestingly, the mercapto-substituted biphenylthiolate SAM on gold (the structure in the middle of Figure 10), a system with essentially no dipole moment along the long molecular axis, showed the work function to be decreased by 1.0 eV when compared to that of clean gold. This has been attributed to the dipole layer formed by the charge rearrangement due to the Au – S bond formation, and labeled a bond dipole. Analysis of the charge-density distribution led the authors to conclude that the bond dipole in all three studied systems is the same and, thus, that the charge rearrangement due to Au – S binding does not depend on the end-substituent of the biphenylthiolate backbone. Surprisingly, the offsets of the highest molecular orbital and the Fermi level for all three systems were calculated to be the same, despite the large differences in the gas-phase molecular ionization potentials. This has serious consequences for tuning of the hole-injection barrier from the gold electrode to the organic monolayer, as the authors clearly showed that the use of SAM constituents with clearly different molecular ionization potentials does not necessarily translate into a different offset between the HOMO and the Fermi level. However, it would be prudent not to draw general conclusions from these findings, as the results were obtained for biphenyl-based systems only and it is not clear if the used DFT methodology describes the studied systems in sufficient detail and accuracy.
Using the same DFT approach as in Ref. 47, Romaner et al. studied the influence of the surface coverage on the work-function changes induced by substituted biphenylthiolate SAMs on gold.48 Though some of the theoretically studied systems are not likely to be synthetically accessible, rather important conclusions were reached. Due to the polarizable nature of the biphenyl backbone of the SAM constituents the molecule – molecule distance on the gold surface has a large effect on the effective dipole-moment-layer depolarization. It was found that the increase of the surface coverage of the biphenylthiolate moieties not only increases the surface density of the dipole moments, but also leads to an increase in molecule – molecule depolarization effects, which effectively decreases the contribution of the additional dipoles to the change of the work function. Also, while the high-coverage systems showed the same behavior as those studied by Heimel et al.,47 at lower coverages there seemed to be a dependence of the energy offset between the HOMO and the Fermi level on the molecular ionization potential of the SAM constituents.
- References
(1)Cahen, D.; Kahn, A. Adv. Mater. 2003, 15, 271-277.
(2)Ishii, H.; Sugiyama, K.; Ito, E.; Seki, K. Adv. Mater. 1999, 11, 605-625.
(3)Heimel, G.; Romaner, L.; Zojer, E.; Bredas, J.-L. Acc. Chem. Res. 2008, 41, 721-729.
(4)Murphy, E. L.; Good, R. H. Phys. Rev. 1956, 102, 1464.
(5)Hölzl, J.; Schulte, F. K.; Wagner, H. Solid surface physics; Springer-Verlag: Berlin, 1979; Vol. 85.
(6)Eastman, D. E. Phys. Rev. B: Condens. Matter 1970, 2, 1.
(7)Bock, C.; Pham, D. V.; Kunze, U.; Kafer, D.; Witte, G.; Woll, C. J. Appl. Phys. 2006, 100, 114517/1-114517/7.
(8)Santato, C.; Cicoira, F.; Cosseddu, P.; Bonfiglio, A.; Bellutti, P.; Muccini, M.; Zamboni, R.; Rosei, F.; Mantoux, A.; Doppelt, P. Appl. Phys. Lett. 2006, 88, 163511/1-163511/3.
(9)Campbell, I. H.; Rubin, S.; Zawodzinski, T. A.; Kress, J. D.; Martin, R. L.; Smith, D. L.; Barashkov, N. N.; Ferraris, J. P. Phys. Rev. B: Condens. Matter 1996, 54, R14321.
(10)De Boer, B.; Hadipour, A.; Mandoc, M. M.; Van Woudenbergh, T.; Blom, P. W. M. Advanced Materials (Weinheim, Germany) 2005, 17, 621-625.
(11)Nieuwenhuys, B. E.; Bouwman, R.; Sachtler, W. M. H. Thin Solid Films 1974, 21, 51-8.
(12)Nieuwenhuys, B. E.; Van Aardenne, O. G.; Sachtler, W. M. H. Chem. Phys. 1974, 5, 418-28.
(13)Huckstadt, C.; Schmidt, S.; Hufner, S.; Forster, F.; Reinert, F.; Springborg, M. Phys. Rev. B: Condens. Matter 2006, 73, 075409/1-075409/10.
(14)Cornil, D.; Olivier, Y.; Geskin, V.; Cornil, J. Adv. Funct. Mater. 2007, 17, 1143-1148.
(15)Romaner, L.; Heimel, G.; Ambrosch-Draxl, C.; Zojer, E. Adv. Funct. Mater. 2008, 18, 3999-4006.
(16)Porter, M. D.; Bright, T. B.; Allara, D. L.; Chidsey, C. E. D. J. Am. Chem. Soc. 1987, 109, 3559-68.
(17)Laibinis, P. E.; Whitesides, G. M.; Allara, D. L.; Tao, Y. T.; Parikh, A. N.; Nuzzo, R. G. J. Am. Chem. Soc. 1991, 113, 7152-67.
(18)Schreiber, F. Prog. Surf. Sci. 2000, 65, 151-257.
(19)Love, J. C.; Estroff, L. A.; Kriebel, J. K.; Nuzzo, R. G.; Whitesides, G. M. Chem. Rev. 2005, 105, 1103-1170.
(20)Laibinis, P. E.; Nuzzo, R. G.; Whitesides, G. M. J. Phys. Chem. 1992, 96, 5097-105.
(21)Duan, L.; Garrett, S. J. J. Phys. Chem. B 2001, 105, 9812-9816.
(22)Kang, J. F.; Ulman, A.; Liao, S.; Jordan, R.; Yang, G.; Liu, G. y. Langmuir 2001, 17, 95-106.
(23)Azzam, W.; Fuxen, C.; Birkner, A.; Rong, H.-T.; Buck, M.; Woell, C. Langmuir 2003, 19, 4958-4968.
(24)Whitesides, G. M.; Laibinis, P. E. Langmuir 1990, 6, 87-96.
(25)Laibinis, P. E.; Bain, C. D.; Whitesides, G. M. J. Phys. Chem. 1991, 95, 7017-7021.
(26)Bumm, L. A.; Arnold, J. J.; Cygan, M. T.; Dunbar, T. D.; Burgin, T. P.; Jones, L., II; Allara, D. L.; Tour, J. M.; Weiss, P. S. Science (Washington, D. C.) 1996, 271, 1705-07.
(27)Zangmeister, C. D.; Robey, S. W.; Van Zee, R. D.; Yao, Y.; Tour, J. M. J. Phys. Chem. B 2004, 108, 16187-16193.
(28)Houseman, B. T.; Gawalt, E. S.; Mrksich, M. Langmuir 2003, 19, 1522-1531.
(29)Lee, J. K.; Lee, K.-B.; Kim, D. J.; Choi, I. S. Langmuir 2003, 19, 8141-8143.
(30)Collman, J. P.; Devaraj, N. K.; Chidsey, C. E. D. Langmuir 2004, 20, 1051-1053.
(31)Evans, S. D.; Ulman, A. Chem. Phys. Lett. 1990, 170, 462-466.
(32)Evans, S. D.; Urankar, E.; Ulman, A.; Ferris, N. J. Am. Chem. Soc. 1991, 113, 4121-4131.
(33)Lu, J.; Delamarche, E.; Eng, L.; Bennewitz, R.; Meyer, E.; Guntherodt, H. J. Langmuir 1999, 15, 8184-8188.
(34)Alloway, D. M.; Hofmann, M.; Smith, D. L.; Gruhn, N. E.; Graham, A. L.; Colorado, R.; Wysocki, V. H.; Lee, T. R.; Lee, P. A.; Armstrong, N. R. J. Phys. Chem. B 2003, 107, 11690-11699.
(35)Wold, D. J.; Frisbie, C. D. J. Am. Chem. Soc. 2001, 123, 5549-5556.
(36)Fuxen, C.; Azzam, W.; Arnold, R.; Witte, G.; Terfort, A.; Woell, C. Langmuir 2001, 17, 3689-3695.
(37)Dhirani, A.-A.; Zehner, R. W.; Hsung, R. P.; Guyot-Sionnest, P.; Sita, L. R. J. Am. Chem. Soc. 1996, 118, 3319-3320.
(38)Yang, G.; Qian, Y.; Engtrakul, C.; Sita, L. R.; Liu, G. y. J. Phys. Chem. B 2000, 104, 9059-9062.
(39)Richter, L. J.; Yang, C. S. C.; Wilson, P. T.; Hacker, C. A.; van Zee, R. D.; Stapleton, J. J.; Allara, D. L.; Yao, Y.; Tour, J. M. J. Phys. Chem. B 2004, 108, 12547-12559.
(40)Arnold, R.; Terfort, A.; Woell, C. Langmuir 2001, 17, 4980-4989.
(41)Frey, S.; Stadler, V.; Heister, K.; Eck, W.; Zharnikov, M.; Grunze, M.; Zeysing, B.; Terfort, A. Langmuir 2001, 17, 2408-2415.
(42)Campbell, I. H.; Kress, J. D.; Martin, R. L.; Smith, D. L.; Barashkov, N. N.; Ferraris, J. P. Appl. Phys. Lett. 1997, 71, 3528-3530.
(43)Zehner, R. W.; Parsons, B. F.; Hsung, R. P.; Sita, L. R. Langmuir 1999, 15, 1121-1127.
(44)Chen, W.; Huang, C.; Gao, X. Y.; Wang, L.; Zhen, C. G.; Qi, D.; Chen, S.; Zhang, H. L.; Loh, K. P.; Chen, Z. K.; Wee, A. T. S. J. Phys. Chem. B 2006, 110, 26075-26080.
(45)Zangmeister, C. D.; Picraux, L. B.; van Zee, R. D.; Yao, Y.; Tour, J. M. Chem. Phys. Lett. 2007, 442, 390-393.
(46)Risko, C.; Zangmeister, C. D.; Yao, Y.; Marks, T. J.; Tour, J. M.; Ratner, M. A.; van Zee, R. D. Journal of Physical Chemistry C 2008, 112, 13215-13225.
(47)Heimel, G.; Romaner, L.; Bredas, J.-L.; Zojer, E. Phys. Rev. Lett. 2006, 96, 196806/1-196806/4.
(48)Romaner, L.; Heimel, G.; Zojer, E. Phys. Rev. B: Condens. Matter 2008, 77, 045113-9.
- ↑ Ishii, H.; Sugiyama, K.; Ito, E.; Seki, K. Adv. Mater. 1999, 11, 605-625
- ↑ Heimel, G.; Romaner, L.; Zojer, E.; Bredas, J.-L. Acc. Chem. Res. 2008, 41, 721-729.
- ↑ Huckstadt, C.; Schmidt, S.; Hufner, S.; Forster, F.; Reinert, F.; Springborg, M. Phys. Rev. B: Condens. Matter 2006, 73, 075409/1-075409/10..