The Polyene Series
Return to Band Structure Menu Next Topic
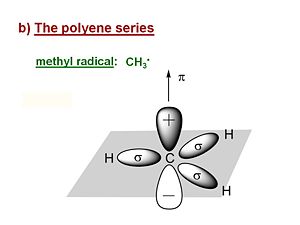
methyl radical
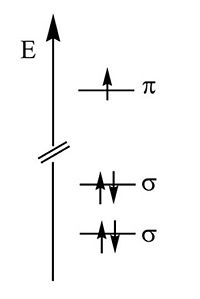
Now we will explore the electronic properties of the polyenes; methylene, ethylene, butadiene etc. Its useful first to look at the methyl radical CH3·. It has a planar structure with the carbon and three of the four valence in the plane. The hydrogens are in sigma orbitals which are symmetrical about the plane of the bond. The unpaired electron or radical is in a 2pz atomic orbital that is perpendicular to the plane of the molecule. The pi AO for that radical has a lobe above the plane, and a negative lobe below the plane. The probability of the electron is always zero at the nucleus. The lobes in the diagram represent the surface within which there is an 80% probability of finding the electron.
The energy of the π electronic orbital is much higher than the σ orbitals. All of the optical and electrical properties of the polyenes are due to highest occupied molecular orbitals which are the π orbitals.
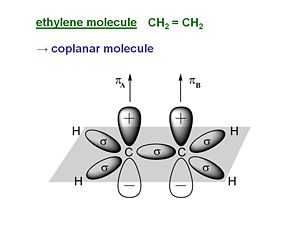
ethylene
Ethylene is pi conjugated molecule. It can be thought of as two methyl radicals coming together and losing two hydrogens. It is coplanar molecule with a sigma bond between the carbons. Each carbon has a pi electron orbital that overlaps with the other because the carbons are close. In a perfectly planar system the pi electrons will not interact with the sigma bond. But as soon as the molecular becomes non coplanar there can be interaction.
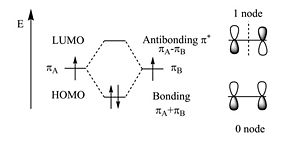
If both pi wavefunctions are the same sign you get a bonding molecular orbital with a higher density between the carbon nuclei (no nodes). If the sign are different you get an antibonding orbital with a node between the nuclei. From a methyl radical like π atomic orbital contribute two pi electrons in to the bonding molecular orbital which will be the highest occupied molecular orbital (HOMO). The antibonding Π * (* denotes unoccupied) corresponds to the lowest unoccupied molecular orbital (LUMO). The energy difference between HOMO and LUMO is 7ev. This is the energy that would have to be supplied by a photon to bring promote an electron to the LUMO level. The visible part of the EM spectrum is between 1.5eV and 3eV. So the first optical transition occurs above the visible spectrum.