Nanocrystalline - Dye Solar Cell Lab
| K-12 Outreach Kits and Labs |
This page is devoted to the research and development of a organic solar cell that can be built in a high school chemistry lab. It is adapted from the nanocrystalline solar cell kit from ICE.
Background
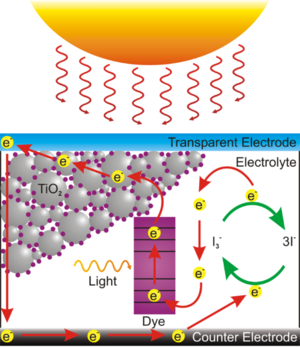
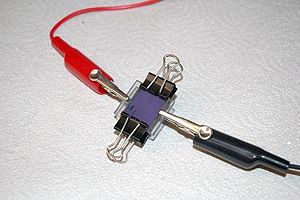
This dye sensitized solar cell, also known as a Graetzel cell uses a thin film of titanium dioxide which has been ground to to a fine powder (nanocrystalline) to increase its reactive surface area. The TiO2 is sandwiched between two glass slides that coated with conductive and transparent indium tin oxide (ITO). The TiO2 is impregnated with some kind of colored dye, in this case anthocyanin from raspberry juice, which is the chemical which first traps the solar energy and passes the charge to the TiO2. Finally the space between the slides is filled with an liquid electrolyte solution of potassium iodide which forms an serves to transport charge (by way of a redox reaction) from the bottom electrode to the dye to complete the circuit.
see wikipedia Dye Sensitized solar cells
Procedure
<embed_document width="55%" height="400">http://depts.washington.edu/cmditr/media/Materials_List.pdf</embed_document>
<embed_document width="55%" height="400">http://depts.washington.edu/cmditr/media/Safety.pdf</embed_document>
<embed_document width="55%" height="400">http://depts.washington.edu/cmditr/media/Color_lab_manual.pdf</embed_document>
Video Instructions
Source for materials
Institute for Chemical Education - Nanocyrstaline Solar Cell Kit
Web Links
Alternative Device Instructions